Expo
view channel
view channel
view channel
view channel
view channel
view channel
view channel
view channel
view channel
Clinical Chem.Molecular DiagnosticsHematologyImmunologyMicrobiologyPathologyTechnologyIndustry
Events
Webinars

- Electronic Nose Smells Early Signs of Ovarian Cancer in Blood
- Simple Blood Test Offers New Path to Alzheimer’s Assessment in Primary Care
- Existing Hospital Analyzers Can Identify Fake Liquid Medical Products
- Rapid Blood Testing Method Aids Safer Decision-Making in Drug-Related Emergencies
- New PSA-Based Prognostic Model Improves Prostate Cancer Risk Assessment
- Blood Test Predicts Which Bladder Cancer Patients May Safely Skip Surgery
- Ultra-Sensitive DNA Test Identifies Relapse Risk in Aggressive Leukemia
- Blood Test Could Help Detect Gallbladder Cancer Earlier
- New Blood Test Score Detects Hidden Alcohol-Related Liver Disease
- New Blood Test Predicts Who Will Most Likely Live Longer
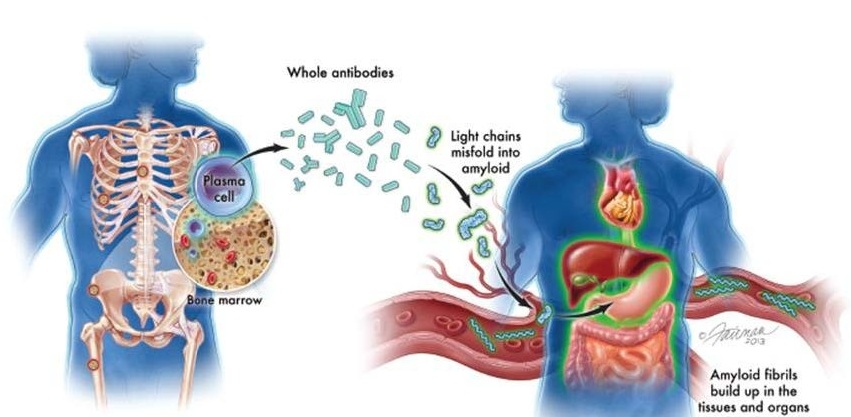
- New Guidelines Aim to Improve AL Amyloidosis Diagnosis
- Fast and Easy Test Could Revolutionize Blood Transfusions
- Automated Hemostasis System Helps Labs of All Sizes Optimize Workflow
- High-Sensitivity Blood Test Improves Assessment of Clotting Risk in Heart Disease Patients
- AI Algorithm Effectively Distinguishes Alpha Thalassemia Subtypes
- Blood Test Identifies Lung Cancer Patients Who Can Benefit from Immunotherapy Drug
- Whole-Genome Sequencing Approach Identifies Cancer Patients Benefitting From PARP-Inhibitor Treatment
- Ultrasensitive Liquid Biopsy Demonstrates Efficacy in Predicting Immunotherapy Response
- Blood Test Could Identify Colon Cancer Patients to Benefit from NSAIDs
- Blood Test Could Detect Adverse Immunotherapy Effects
- Hidden Gut Viruses Linked to Colorectal Cancer Risk
- Three-Test Panel Launched for Detection of Liver Fluke Infections
- Rapid Test Promises Faster Answers for Drug-Resistant Infections
- CRISPR-Based Technology Neutralizes Antibiotic-Resistant Bacteria
- Comprehensive Review Identifies Gut Microbiome Signatures Associated With Alzheimer’s Disease
- AI-Powered Biomarker Predicts Liver Cancer Risk
- Robotic Technology Unveiled for Automated Diagnostic Blood Draws
- ADLM Launches First-of-Its-Kind Data Science Program for Laboratory Medicine Professionals
- Aptamer Biosensor Technology to Transform Virus Detection
- AI Models Could Predict Pre-Eclampsia and Anemia Earlier Using Routine Blood Tests
- QuidelOrtho Collaborates with Lifotronic to Expand Global Immunoassay Portfolio
- WHX Labs in Dubai spotlights leadership skills shaping next-generation laboratories
- New Collaboration Brings Automated Mass Spectrometry to Routine Laboratory Testing
- AI-Powered Cervical Cancer Test Set for Major Rollout in Latin America
- Diasorin and Fisher Scientific Enter into US Distribution Agreement for Molecular POC Platform
- Protective Brain Protein Emerges as Biomarker Target in Alzheimer’s Disease
- Genome Analysis Predicts Likelihood of Neurodisability in Oxygen-Deprived Newborns
- Gene Panel Predicts Disease Progession for Patients with B-cell Lymphoma
- New Method Simplifies Preparation of Tumor Genomic DNA Libraries
- New Tool Developed for Diagnosis of Chronic HBV Infection
- Urine Specimen Collection System Improves Diagnostic Accuracy and Efficiency
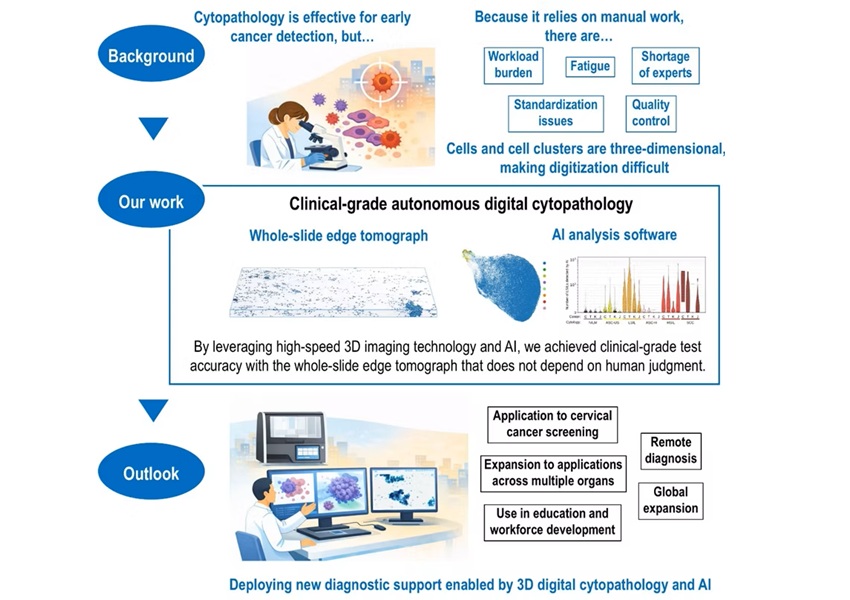
- AI-Powered 3D Scanning System Speeds Cancer Screening
- Single Sample Classifier Predicts Cancer-Associated Fibroblast Subtypes in Patient Samples
- New AI-Driven Platform Standardizes Tuberculosis Smear Microscopy Workflow
- AI Tool Uses Blood Biomarkers to Predict Transplant Complications Before Symptoms Appear

 Expo
Expo
- Electronic Nose Smells Early Signs of Ovarian Cancer in Blood
- Simple Blood Test Offers New Path to Alzheimer’s Assessment in Primary Care
- Existing Hospital Analyzers Can Identify Fake Liquid Medical Products
- Rapid Blood Testing Method Aids Safer Decision-Making in Drug-Related Emergencies
- New PSA-Based Prognostic Model Improves Prostate Cancer Risk Assessment
- Blood Test Predicts Which Bladder Cancer Patients May Safely Skip Surgery
- Ultra-Sensitive DNA Test Identifies Relapse Risk in Aggressive Leukemia
- Blood Test Could Help Detect Gallbladder Cancer Earlier
- New Blood Test Score Detects Hidden Alcohol-Related Liver Disease
- New Blood Test Predicts Who Will Most Likely Live Longer
- New Guidelines Aim to Improve AL Amyloidosis Diagnosis
- Fast and Easy Test Could Revolutionize Blood Transfusions
- Automated Hemostasis System Helps Labs of All Sizes Optimize Workflow
- High-Sensitivity Blood Test Improves Assessment of Clotting Risk in Heart Disease Patients
- AI Algorithm Effectively Distinguishes Alpha Thalassemia Subtypes
- Blood Test Identifies Lung Cancer Patients Who Can Benefit from Immunotherapy Drug
- Whole-Genome Sequencing Approach Identifies Cancer Patients Benefitting From PARP-Inhibitor Treatment
- Ultrasensitive Liquid Biopsy Demonstrates Efficacy in Predicting Immunotherapy Response
- Blood Test Could Identify Colon Cancer Patients to Benefit from NSAIDs
- Blood Test Could Detect Adverse Immunotherapy Effects
- Hidden Gut Viruses Linked to Colorectal Cancer Risk
- Three-Test Panel Launched for Detection of Liver Fluke Infections
- Rapid Test Promises Faster Answers for Drug-Resistant Infections
- CRISPR-Based Technology Neutralizes Antibiotic-Resistant Bacteria
- Comprehensive Review Identifies Gut Microbiome Signatures Associated With Alzheimer’s Disease
- AI-Powered Biomarker Predicts Liver Cancer Risk
- Robotic Technology Unveiled for Automated Diagnostic Blood Draws
- ADLM Launches First-of-Its-Kind Data Science Program for Laboratory Medicine Professionals
- Aptamer Biosensor Technology to Transform Virus Detection
- AI Models Could Predict Pre-Eclampsia and Anemia Earlier Using Routine Blood Tests
- QuidelOrtho Collaborates with Lifotronic to Expand Global Immunoassay Portfolio
- WHX Labs in Dubai spotlights leadership skills shaping next-generation laboratories
- New Collaboration Brings Automated Mass Spectrometry to Routine Laboratory Testing
- AI-Powered Cervical Cancer Test Set for Major Rollout in Latin America
- Diasorin and Fisher Scientific Enter into US Distribution Agreement for Molecular POC Platform
- Protective Brain Protein Emerges as Biomarker Target in Alzheimer’s Disease
- Genome Analysis Predicts Likelihood of Neurodisability in Oxygen-Deprived Newborns
- Gene Panel Predicts Disease Progession for Patients with B-cell Lymphoma
- New Method Simplifies Preparation of Tumor Genomic DNA Libraries
- New Tool Developed for Diagnosis of Chronic HBV Infection
- Urine Specimen Collection System Improves Diagnostic Accuracy and Efficiency
- AI-Powered 3D Scanning System Speeds Cancer Screening
- Single Sample Classifier Predicts Cancer-Associated Fibroblast Subtypes in Patient Samples
- New AI-Driven Platform Standardizes Tuberculosis Smear Microscopy Workflow
- AI Tool Uses Blood Biomarkers to Predict Transplant Complications Before Symptoms Appear