Expo
view channel
view channel
view channel
view channel
view channel
view channel
view channel
view channel
view channel
Clinical Chem.
HematologyImmunologyMicrobiologyPathologyTechnologyIndustry
Events
Webinars

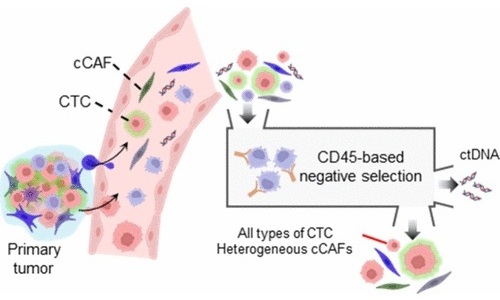
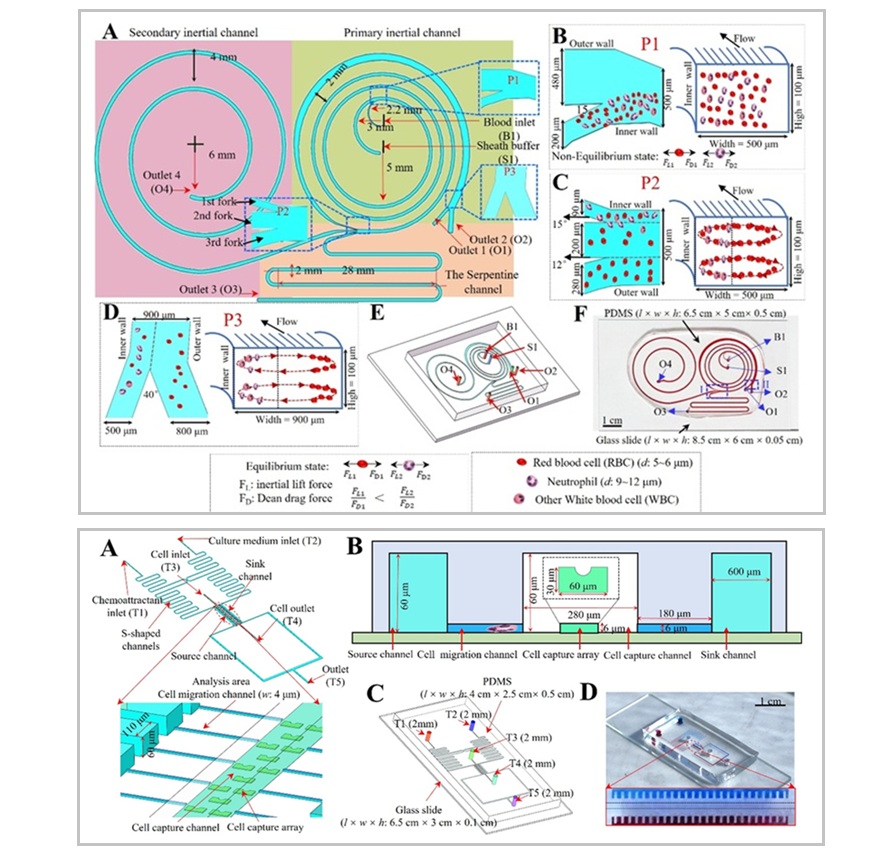
- Simultaneous Cell Isolation Technology Improves Cancer Diagnostic Accuracy
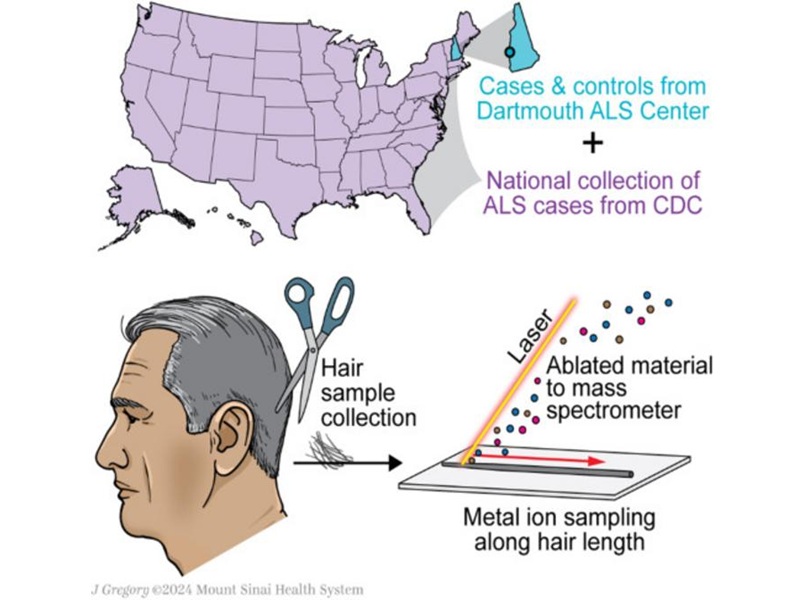
- Simple Non-Invasive Hair-Based Test Could Speed ALS Diagnosis
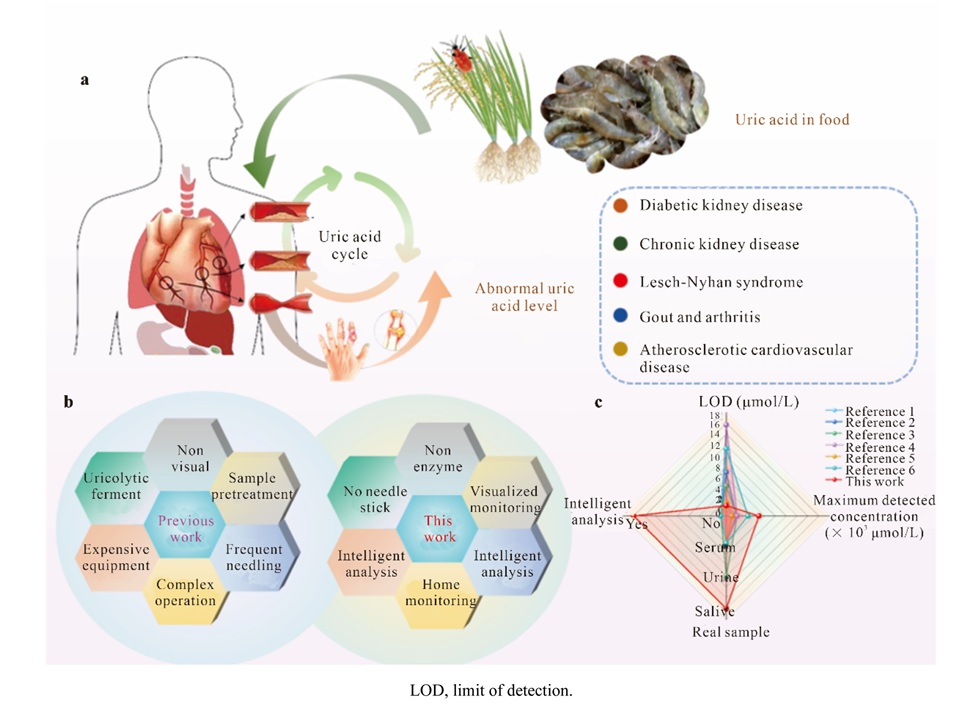
- Paper Strip Saliva Test Detects Elevated Uric Acid Levels Without Blood Draws
- Prostate Cancer Markers Based on Chemical Make-Up of Calcifications to Speed Up Detection
- Breath Test Could Help Detect Blood Cancers
- Automated High Throughput Immunoassay Test to Advance Neurodegenerative Clinical Research
- Ultrasensitive Test Could Identify Earliest Molecular Signs of Metastatic Relapse in Breast Cancer Patients
- Blood Test Could Detect Proteins Linked to Alzheimer's Disease and Memory Loss
- Brain Inflammation Biomarker Detects Alzheimer’s Years Before Symptoms Appear
- First-of-Its-Kind Blood Test Detects Over 50 Cancer Types
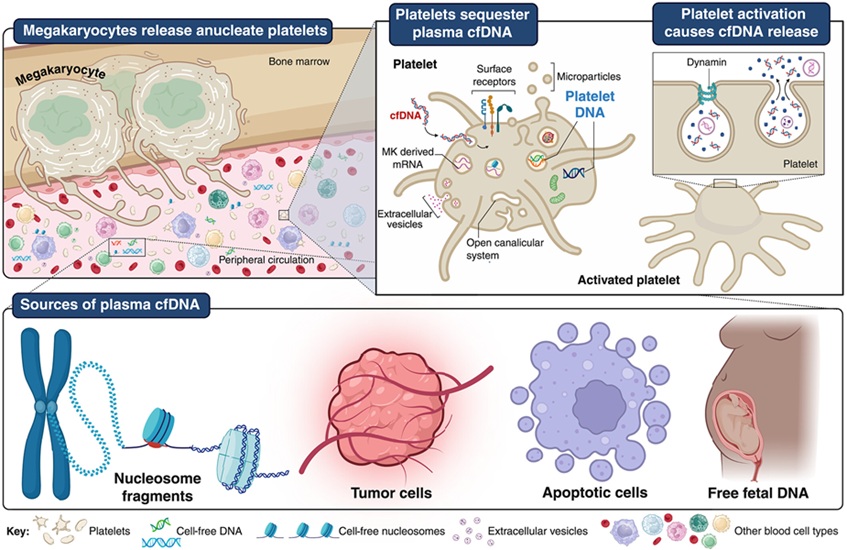
- Platelets Could Improve Early and Minimally Invasive Detection of Cancer
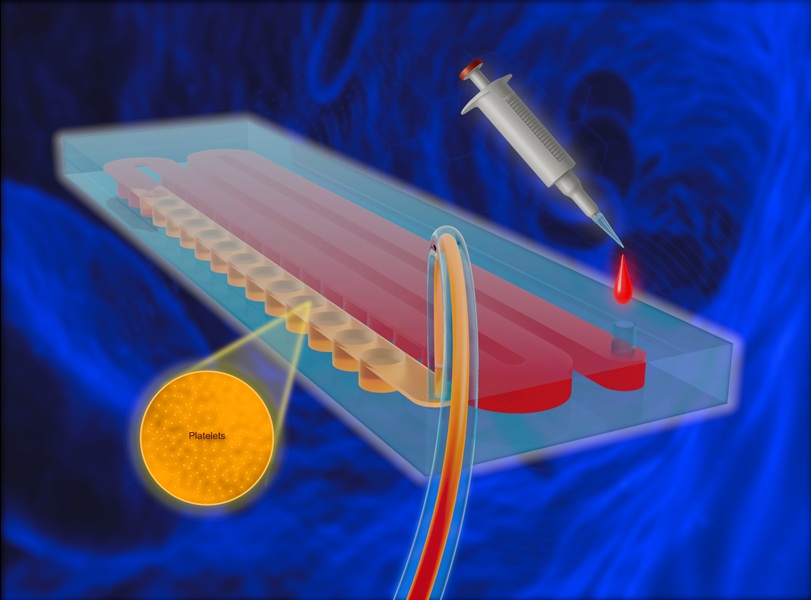
- Portable and Disposable Device Obtains Platelet-Rich Plasma Without Complex Equipment
- Disposable Cartridge-Based Test Delivers Rapid and Accurate CBC Results
- First Point-of-Care Heparin Monitoring Test Provides Results in Under 15 Minutes
- New Scoring System Predicts Risk of Developing Cancer from Common Blood Disorder
- Companion Diagnostic Test Identifies HER2-Ultralow Breast Cancer and Biliary Tract Cancer Patients
- Novel Multiplex Assay Supports Diagnosis of Autoimmune Vasculitis
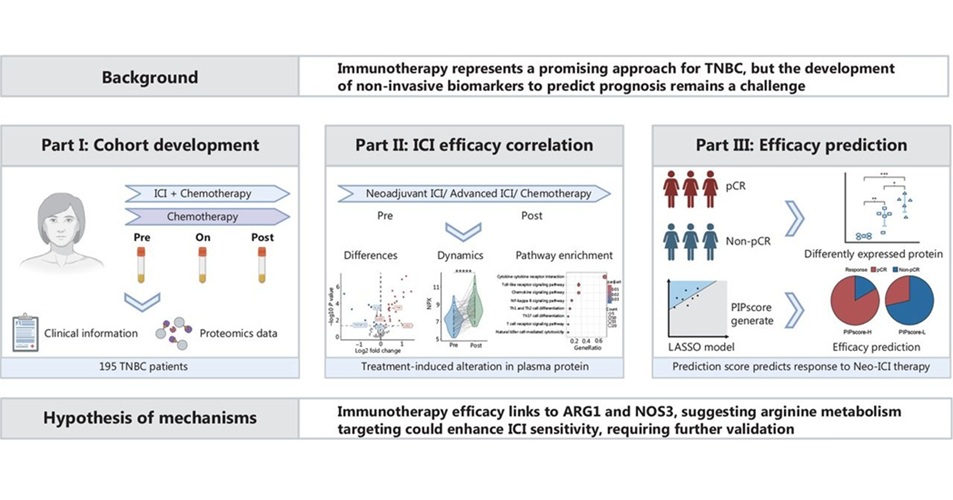
- Blood Test Predicts Immunotherapy Efficacy in Triple-Negative Breast Cancer
- Simple Genetic Testing Could Predict Treatment Success in Multiple Sclerosis Patients
- Novel Gene Signature Predicts Immunotherapy Response in Advanced Kidney Cancers
- New Diagnostic Method Confirms Sepsis Infections Earlier
- New Markers Could Predict Risk of Severe Chlamydia Infection
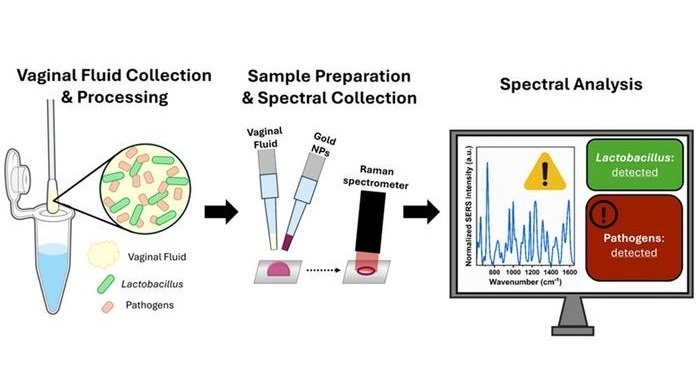
- Portable Spectroscopy Rapidly and Noninvasively Detects Bacterial Species in Vaginal Fluid
- CRISPR-Based Saliva Test Detects Tuberculosis Directly from Sputum
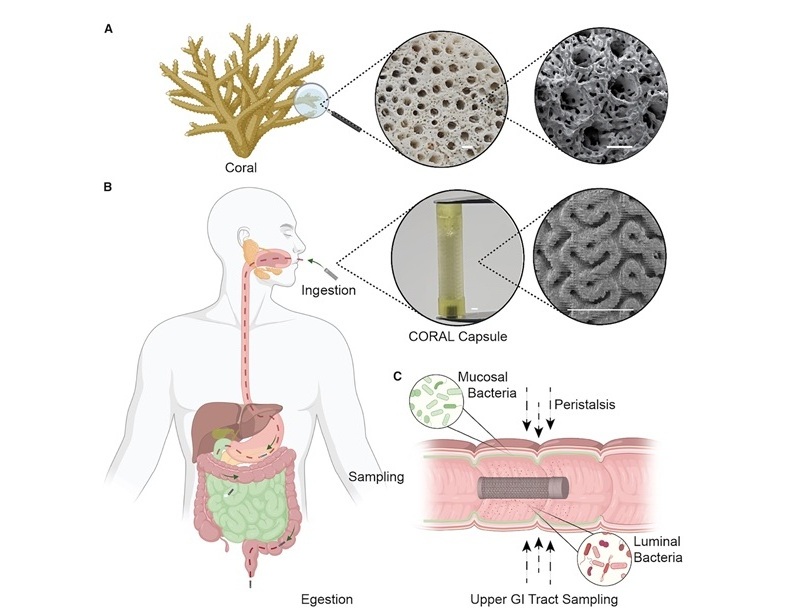
- Urine-Based Assay Diagnoses Common Lung Infection in Immunocompromised People
- Rapid Diagnostic Technology Utilizes Breath Samples to Detect Lower Respiratory Tract Infections
- Graphene-Based Sensor Uses Breath Sample to Identify Diabetes and Prediabetes in Minutes
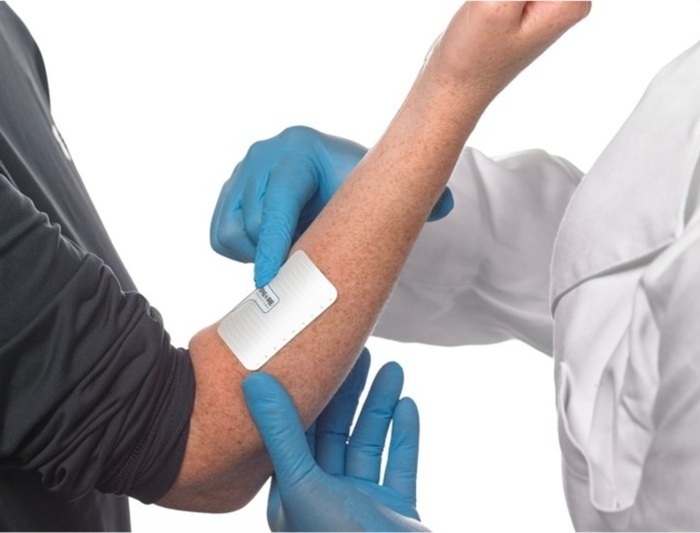
- Wireless Sweat Patch Could Be Used as Diagnostic Test for Cystic Fibrosis
- New Method Advances AI Reliability with Applications in Medical Diagnostics
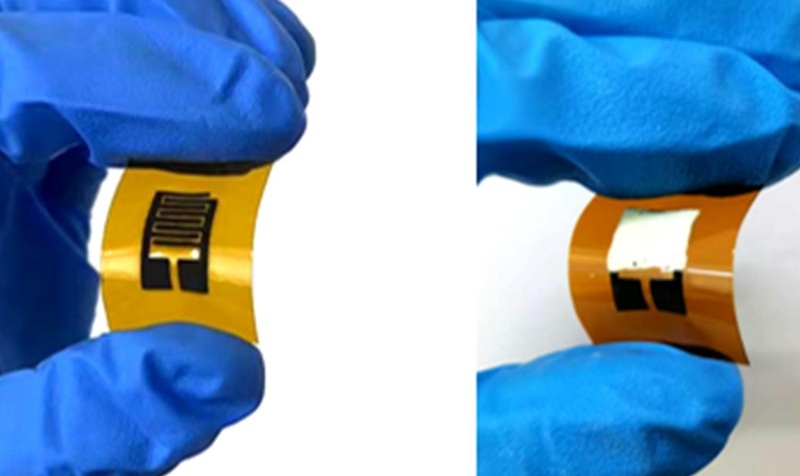
- Self-Powered Microneedle Patch Collects Biomarker Samples Without Drawing Blood
- Werfen and VolitionRx Partner to Advance Diagnostic Testing for Antiphospholipid Syndrome
- Siemens Healthineers and Carna Health Partner to Advance Kidney Care Innovation
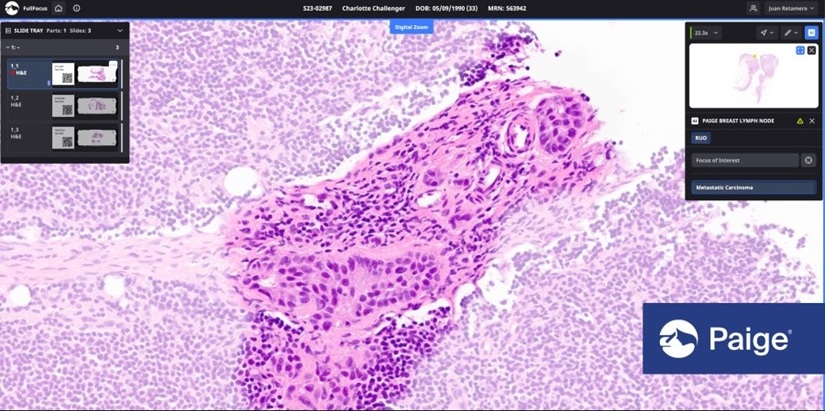
- Tempus AI Acquires Digital Pathology Company Paige
- Vircell Launches New Website for Molecular Control Products
- New Manual to Help Clinicians Better Diagnose and Treat Infection-Associated Chronic Illness
- Gene Panel Predicts Disease Progession for Patients with B-cell Lymphoma
- New Method Simplifies Preparation of Tumor Genomic DNA Libraries
- New Tool Developed for Diagnosis of Chronic HBV Infection
- Panel of Genetic Loci Accurately Predicts Risk of Developing Gout
- Disrupted TGFB Signaling Linked to Increased Cancer-Related Bacteria
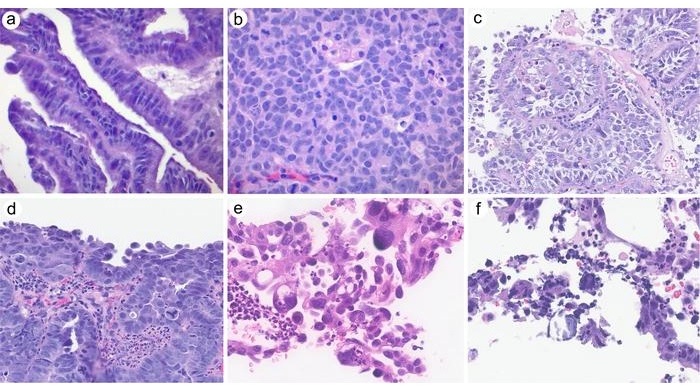
- Clinicopathologic Study Supports Exclusion of Cervical Serous Carcinoma from WHO Classification
- Mobile-Compatible AI-Powered System to Revolutionize Malaria Diagnosis
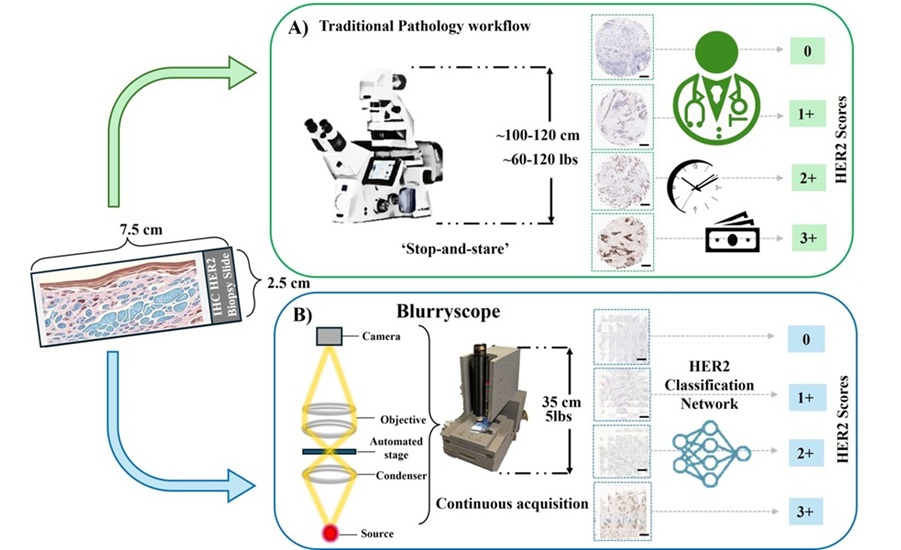
- Compact AI-Powered Microscope Enables Rapid Cost-Effective Cancer Scoring
- New Method Enables Precise Detection of Nanoplastics in Body
- AI-Powered Tool Improves Cancer Tissue Analysis

 Expo
Expo
- Simultaneous Cell Isolation Technology Improves Cancer Diagnostic Accuracy
- Simple Non-Invasive Hair-Based Test Could Speed ALS Diagnosis
- Paper Strip Saliva Test Detects Elevated Uric Acid Levels Without Blood Draws
- Prostate Cancer Markers Based on Chemical Make-Up of Calcifications to Speed Up Detection
- Breath Test Could Help Detect Blood Cancers
- Automated High Throughput Immunoassay Test to Advance Neurodegenerative Clinical Research
- Ultrasensitive Test Could Identify Earliest Molecular Signs of Metastatic Relapse in Breast Cancer Patients
- Blood Test Could Detect Proteins Linked to Alzheimer's Disease and Memory Loss
- Brain Inflammation Biomarker Detects Alzheimer’s Years Before Symptoms Appear
- First-of-Its-Kind Blood Test Detects Over 50 Cancer Types
- Platelets Could Improve Early and Minimally Invasive Detection of Cancer
- Portable and Disposable Device Obtains Platelet-Rich Plasma Without Complex Equipment
- Disposable Cartridge-Based Test Delivers Rapid and Accurate CBC Results
- First Point-of-Care Heparin Monitoring Test Provides Results in Under 15 Minutes
- New Scoring System Predicts Risk of Developing Cancer from Common Blood Disorder
- Companion Diagnostic Test Identifies HER2-Ultralow Breast Cancer and Biliary Tract Cancer Patients
- Novel Multiplex Assay Supports Diagnosis of Autoimmune Vasculitis
- Blood Test Predicts Immunotherapy Efficacy in Triple-Negative Breast Cancer
- Simple Genetic Testing Could Predict Treatment Success in Multiple Sclerosis Patients
- Novel Gene Signature Predicts Immunotherapy Response in Advanced Kidney Cancers
- New Diagnostic Method Confirms Sepsis Infections Earlier
- New Markers Could Predict Risk of Severe Chlamydia Infection
- Portable Spectroscopy Rapidly and Noninvasively Detects Bacterial Species in Vaginal Fluid
- CRISPR-Based Saliva Test Detects Tuberculosis Directly from Sputum
- Urine-Based Assay Diagnoses Common Lung Infection in Immunocompromised People
- Rapid Diagnostic Technology Utilizes Breath Samples to Detect Lower Respiratory Tract Infections
- Graphene-Based Sensor Uses Breath Sample to Identify Diabetes and Prediabetes in Minutes
- Wireless Sweat Patch Could Be Used as Diagnostic Test for Cystic Fibrosis
- New Method Advances AI Reliability with Applications in Medical Diagnostics
- Self-Powered Microneedle Patch Collects Biomarker Samples Without Drawing Blood
- Werfen and VolitionRx Partner to Advance Diagnostic Testing for Antiphospholipid Syndrome
- Siemens Healthineers and Carna Health Partner to Advance Kidney Care Innovation
- Tempus AI Acquires Digital Pathology Company Paige
- Vircell Launches New Website for Molecular Control Products
- New Manual to Help Clinicians Better Diagnose and Treat Infection-Associated Chronic Illness
- Gene Panel Predicts Disease Progession for Patients with B-cell Lymphoma
- New Method Simplifies Preparation of Tumor Genomic DNA Libraries
- New Tool Developed for Diagnosis of Chronic HBV Infection
- Panel of Genetic Loci Accurately Predicts Risk of Developing Gout
- Disrupted TGFB Signaling Linked to Increased Cancer-Related Bacteria
- Clinicopathologic Study Supports Exclusion of Cervical Serous Carcinoma from WHO Classification
- Mobile-Compatible AI-Powered System to Revolutionize Malaria Diagnosis
- Compact AI-Powered Microscope Enables Rapid Cost-Effective Cancer Scoring
- New Method Enables Precise Detection of Nanoplastics in Body
- AI-Powered Tool Improves Cancer Tissue Analysis