Expo
WHX Labs Dubai
view channel
view channel
view channel
view channel
view channel
view channel
view channel
view channel
view channel
Clinical Chem.
HematologyImmunologyMicrobiologyPathologyTechnologyIndustry
Events
Webinars

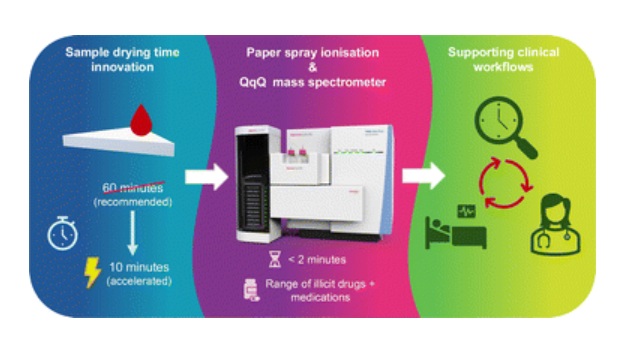
- Rapid Blood Testing Method Aids Safer Decision-Making in Drug-Related Emergencies
- New PSA-Based Prognostic Model Improves Prostate Cancer Risk Assessment
- Extracellular Vesicles Linked to Heart Failure Risk in CKD Patients
- Study Compares Analytical Performance of Quantitative Hepatitis B Surface Antigen Assays
- Blood Test Could Predict and Identify Early Relapses in Myeloma Patients
- AI-Powered Liquid Biopsy Classifies Pediatric Brain Tumors with High Accuracy
- New CSF Liquid Biopsy Assay Reveals Genomic Insights for CNS Tumors
- Group A Strep Molecular Test Delivers Definitive Results at POC in 15 Minutes
- Rapid Molecular Test Identifies Sepsis Patients Most Likely to Have Positive Blood Cultures
- Light-Based Sensor Detects Early Molecular Signs of Cancer in Blood
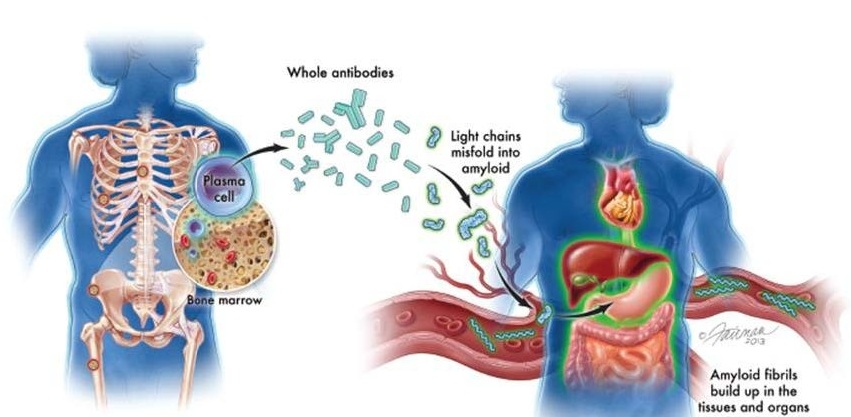
- New Guidelines Aim to Improve AL Amyloidosis Diagnosis
- Fast and Easy Test Could Revolutionize Blood Transfusions
- Automated Hemostasis System Helps Labs of All Sizes Optimize Workflow
- High-Sensitivity Blood Test Improves Assessment of Clotting Risk in Heart Disease Patients
- AI Algorithm Effectively Distinguishes Alpha Thalassemia Subtypes
- Blood Test Identifies Lung Cancer Patients Who Can Benefit from Immunotherapy Drug
- Whole-Genome Sequencing Approach Identifies Cancer Patients Benefitting From PARP-Inhibitor Treatment
- Ultrasensitive Liquid Biopsy Demonstrates Efficacy in Predicting Immunotherapy Response
- Blood Test Could Identify Colon Cancer Patients to Benefit from NSAIDs
- Blood Test Could Detect Adverse Immunotherapy Effects
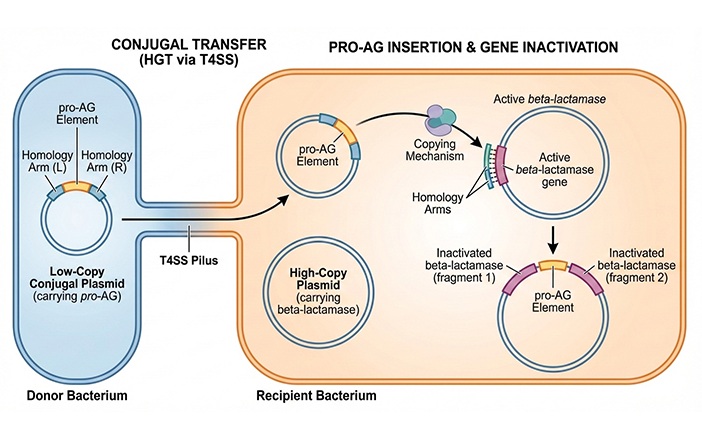
- CRISPR-Based Technology Neutralizes Antibiotic-Resistant Bacteria
- Comprehensive Review Identifies Gut Microbiome Signatures Associated With Alzheimer’s Disease
- AI-Powered Platform Enables Rapid Detection of Drug-Resistant C. Auris Pathogens
- New Test Measures How Effectively Antibiotics Kill Bacteria
- New Antimicrobial Stewardship Standards for TB Care to Optimize Diagnostics
- AI-Powered Biomarker Predicts Liver Cancer Risk
- Robotic Technology Unveiled for Automated Diagnostic Blood Draws
- ADLM Launches First-of-Its-Kind Data Science Program for Laboratory Medicine Professionals
- Aptamer Biosensor Technology to Transform Virus Detection
- AI Models Could Predict Pre-Eclampsia and Anemia Earlier Using Routine Blood Tests
- WHX Labs in Dubai spotlights leadership skills shaping next-generation laboratories
- New Collaboration Brings Automated Mass Spectrometry to Routine Laboratory Testing
- AI-Powered Cervical Cancer Test Set for Major Rollout in Latin America
- Diasorin and Fisher Scientific Enter into US Distribution Agreement for Molecular POC Platform
- WHX Labs Dubai to Gather Global Experts in Antimicrobial Resistance at Inaugural AMR Leaders’ Summit
- Gene Panel Predicts Disease Progession for Patients with B-cell Lymphoma
- New Method Simplifies Preparation of Tumor Genomic DNA Libraries
- New Tool Developed for Diagnosis of Chronic HBV Infection
- Panel of Genetic Loci Accurately Predicts Risk of Developing Gout
- Disrupted TGFB Signaling Linked to Increased Cancer-Related Bacteria
- New AI-Driven Platform Standardizes Tuberculosis Smear Microscopy Workflow
- AI Tool Uses Blood Biomarkers to Predict Transplant Complications Before Symptoms Appear
- Research Consortium Harnesses AI and Spatial Biology to Advance Cancer Discovery
- High-Resolution Cancer Virus Imaging Uncovers Potential Therapeutic Targets
- AI Tool Helps See How Cells Work Together Inside Diseased Tissue

 Expo
WHX Labs Dubai
Expo
WHX Labs Dubai
- Rapid Blood Testing Method Aids Safer Decision-Making in Drug-Related Emergencies
- New PSA-Based Prognostic Model Improves Prostate Cancer Risk Assessment
- Extracellular Vesicles Linked to Heart Failure Risk in CKD Patients
- Study Compares Analytical Performance of Quantitative Hepatitis B Surface Antigen Assays
- Blood Test Could Predict and Identify Early Relapses in Myeloma Patients
- AI-Powered Liquid Biopsy Classifies Pediatric Brain Tumors with High Accuracy
- New CSF Liquid Biopsy Assay Reveals Genomic Insights for CNS Tumors
- Group A Strep Molecular Test Delivers Definitive Results at POC in 15 Minutes
- Rapid Molecular Test Identifies Sepsis Patients Most Likely to Have Positive Blood Cultures
- Light-Based Sensor Detects Early Molecular Signs of Cancer in Blood
- New Guidelines Aim to Improve AL Amyloidosis Diagnosis
- Fast and Easy Test Could Revolutionize Blood Transfusions
- Automated Hemostasis System Helps Labs of All Sizes Optimize Workflow
- High-Sensitivity Blood Test Improves Assessment of Clotting Risk in Heart Disease Patients
- AI Algorithm Effectively Distinguishes Alpha Thalassemia Subtypes
- Blood Test Identifies Lung Cancer Patients Who Can Benefit from Immunotherapy Drug
- Whole-Genome Sequencing Approach Identifies Cancer Patients Benefitting From PARP-Inhibitor Treatment
- Ultrasensitive Liquid Biopsy Demonstrates Efficacy in Predicting Immunotherapy Response
- Blood Test Could Identify Colon Cancer Patients to Benefit from NSAIDs
- Blood Test Could Detect Adverse Immunotherapy Effects
- CRISPR-Based Technology Neutralizes Antibiotic-Resistant Bacteria
- Comprehensive Review Identifies Gut Microbiome Signatures Associated With Alzheimer’s Disease
- AI-Powered Platform Enables Rapid Detection of Drug-Resistant C. Auris Pathogens
- New Test Measures How Effectively Antibiotics Kill Bacteria
- New Antimicrobial Stewardship Standards for TB Care to Optimize Diagnostics
- AI-Powered Biomarker Predicts Liver Cancer Risk
- Robotic Technology Unveiled for Automated Diagnostic Blood Draws
- ADLM Launches First-of-Its-Kind Data Science Program for Laboratory Medicine Professionals
- Aptamer Biosensor Technology to Transform Virus Detection
- AI Models Could Predict Pre-Eclampsia and Anemia Earlier Using Routine Blood Tests
- WHX Labs in Dubai spotlights leadership skills shaping next-generation laboratories
- New Collaboration Brings Automated Mass Spectrometry to Routine Laboratory Testing
- AI-Powered Cervical Cancer Test Set for Major Rollout in Latin America
- Diasorin and Fisher Scientific Enter into US Distribution Agreement for Molecular POC Platform
- WHX Labs Dubai to Gather Global Experts in Antimicrobial Resistance at Inaugural AMR Leaders’ Summit
- MEDLAB Europe 2018 Presents Latest Technologies and Developments
- Siemens Healthineers Showcases Advanced Diagnostics Analyzers
- BIOBASE Displays Range Of Laboratory & Medical Analyzers
- Fujifilm Showcases DRI-CHEM NX700 Dry Chemistry Analyzer
- MedLab Middle East Continues as World's Largest Attended Laboratory Exhibition and Conference
- Telstar Promotes Latest Generation of Freeze Dryers in Dubai
- Siemens Healthineers Showcases In-Vitro Diagnostics Products at MedLab 2019
- Randox Promotes Evidence MultiSTAT Automated Benchtop Immunoassay Analyzer
- Mindray Displays New Generation Benchtop Cellular Analysis Line
- HORIBA Medical Shows Latest Yumizen G Range at Lab Trade Show
- Gene Panel Predicts Disease Progession for Patients with B-cell Lymphoma
- New Method Simplifies Preparation of Tumor Genomic DNA Libraries
- New Tool Developed for Diagnosis of Chronic HBV Infection
- Panel of Genetic Loci Accurately Predicts Risk of Developing Gout
- Disrupted TGFB Signaling Linked to Increased Cancer-Related Bacteria
- DAS Srl Exhibits Range of Fully Automated Systems at MEDLAB Middle East 2020
- GeneMatrix Promotes Sex and Respiratory Infection Testing Products at MEDLAB
- Mindray Showcases Comprehensive IVD Solutions Portfolio at MEDLAB Middle East 2020
- New Technology that Detects MRSA in 15 Minutes Introduced at MEDLAB Middle East 2020
- Vircell Exhibits Its New VirClia Lotus and qSPEED-OLIGO Systems at MEDLAB
- MP Biomedicals Exhibits Diagnostics Kits and Sample Prep Solutions at MEDLAB Middle East 2021
- BD Demonstrates Latest Medtech Innovations at MEDLAB Middle East 2021
- Diagnostica Stago Highlights Max Generation Family of Coagulation Analyzers at MEDLAB Middle East 2021
- Randox Showcases Latest Product Portfolio and Services at MEDLAB Middle East 2021
- Horiba Medical Exhibits New Generation of Automated Hematology Analyzers at MEDLAB Middle East 2021
- PerkinElmer Exhibits Automated Nucleic Acid Extraction Solutions at MEDLAB Middle East
- Abbott Displays Its Life-Changing POC Tests and Diagnostic Tools at MEDLAB Middle East
- Diagast Presents Latest Immunohaematology Solutions at MEDLAB Middle East
- Siemens Brings Its Latest Innovations in Laboratory Diagnostics to MEDLAB Middle East 2022
- Bioperfectus Showcases Its Molecular Diagnostic Products for Infectious Diseases at MEDLAB 2022 Online
- SNIBE Brings Latest Innovations in Immunoassay, Clinical Biochemistry and Molecular Diagnostics to Medlab Middle East 2023
- Sansure Exhibits Portable Molecular Workstation at Medlab Middle East 2023
- QIAGEN Demonstrates Its Revolutionary Molecular Diagnostic Solution at Medlab Middle East 2023
- QuidelOrtho Demonstrates Automated, Sample-To-Result, Multiplex RT-PCR Testing Platform at Medlab Middle East 2023
- Erba Introduces XL Range of Fully Automated Clinical Chemistry Systems at Medlab Middle East 2023
- 77 Elektronika Showcases Latest Developments in Urinalysis
- BioVendor Group Unveils Latest Innovations in Immunodiagnostics and Molecular Diagnostics
- Sansure Presents Future of Pathogen Identification and Disease Diagnosis
- Spinreact Presents Latest Developments in IVD
- Alifax Demonstrates Automated Solutions to Improve Performance in Laboratory Settings
- BIOBASE Showcases Comprehensive Range of Laboratory Solutions
- Dymind Presents Advanced Automatic Hematology Analysis Line
- BioPerfectus Presents Latest Solutions for Women's Health and Infectious Disease Diagnosis
- YHLO Unveils Total Laboratory Automation System for Elevated Laboratory Efficiency
- Vazyme Showcases Cutting-Edge Microfluidic Solutions for Precise and Efficient Laboratory Workflows
- Experts Highlight Role of Artificial Intelligence in Shaping Healthcare 3.0 at WHX Labs
- General Biologicals Displays CLIA and Molecular Diagnostic Solutions at WHX Labs Dubai
- Anbio Presents Rapid, Accessible POCT Diagnostics to WHX Labs Dubai 2026
- Seegene Showcases Comprehensive Molecular Diagnostics Portfolio at WHX Labs Dubai
- Beckman Coulter Highlights Latest Innovations in Clinical Diagnostics at WHX Labs Dubai
- New AI-Driven Platform Standardizes Tuberculosis Smear Microscopy Workflow
- AI Tool Uses Blood Biomarkers to Predict Transplant Complications Before Symptoms Appear
- Research Consortium Harnesses AI and Spatial Biology to Advance Cancer Discovery
- High-Resolution Cancer Virus Imaging Uncovers Potential Therapeutic Targets
- AI Tool Helps See How Cells Work Together Inside Diseased Tissue