Expo
view channel
view channel
view channel
view channel
view channel
view channel
view channel
view channel
view channel
Clinical Chem.Molecular DiagnosticsHematologyImmunologyMicrobiologyPathologyTechnologyIndustry
Events

- 3D Printed Point-Of-Care Mass Spectrometer Outperforms State-Of-The-Art Models
- POC Biomedical Test Spins Water Droplet Using Sound Waves for Cancer Detection
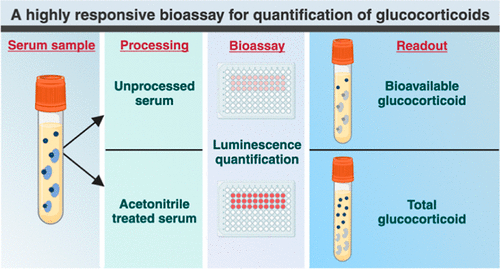
- Highly Reliable Cell-Based Assay Enables Accurate Diagnosis of Endocrine Diseases
- New Blood Testing Method Detects Potent Opioids in Under Three Minutes
- Wireless Hepatitis B Test Kit Completes Screening and Data Collection in One Step
- Blood-Based Test Outperforms Ultrasound in Early Liver Cancer Detection
- Four-In-One Molecular Test Detects and Differentiates Among Most Prevalent Respiratory Viruses in 20 Minutes
- First-Line PSA Testing More Cost-Effective Than First-Line MRI for Prostate Cancer Screening
- Proteomics Platform Identifies Proteins in Blood to Give Cancer Warning 7 Years before Diagnosis
- AI Technology-Based Blood Test Identifies Lung Cancer Earlier
- First 4-in-1 Nucleic Acid Test for Arbovirus Screening to Reduce Risk of Transfusion-Transmitted Infections
- POC Finger-Prick Blood Test Determines Risk of Neutropenic Sepsis in Patients Undergoing Chemotherapy
- First Affordable and Rapid Test for Beta Thalassemia Demonstrates 99% Diagnostic Accuracy
- Handheld White Blood Cell Tracker to Enable Rapid Testing For Infections
- Smart Palm-size Optofluidic Hematology Analyzer Enables POCT of Patients’ Blood Cells
- Molecular Profiling Improves Diagnosis for Children with High Risk Cancers
- Blood Test Measures Immune Response to Epstein-Barr Virus in MS Patients
- AI Predicts Tumor-Killing Cells with High Accuracy
- Diagnostic Blood Test for Cellular Rejection after Organ Transplant Could Replace Surgical Biopsies
- AI Tool Precisely Matches Cancer Drugs to Patients Using Information from Each Tumor Cell
- POC STI Test Shortens Time from ED Arrival to Test Results
- Integrated Solution Ushers New Era of Automated Tuberculosis Testing
- Automated Sepsis Test System Enables Rapid Diagnosis for Patients with Severe Bloodstream Infections
- Enhanced Rapid Syndromic Molecular Diagnostic Solution Detects Broad Range of Infectious Diseases
- Clinical Decision Support Software a Game-Changer in Antimicrobial Resistance Battle
- New Diagnostic System Achieves PCR Testing Accuracy
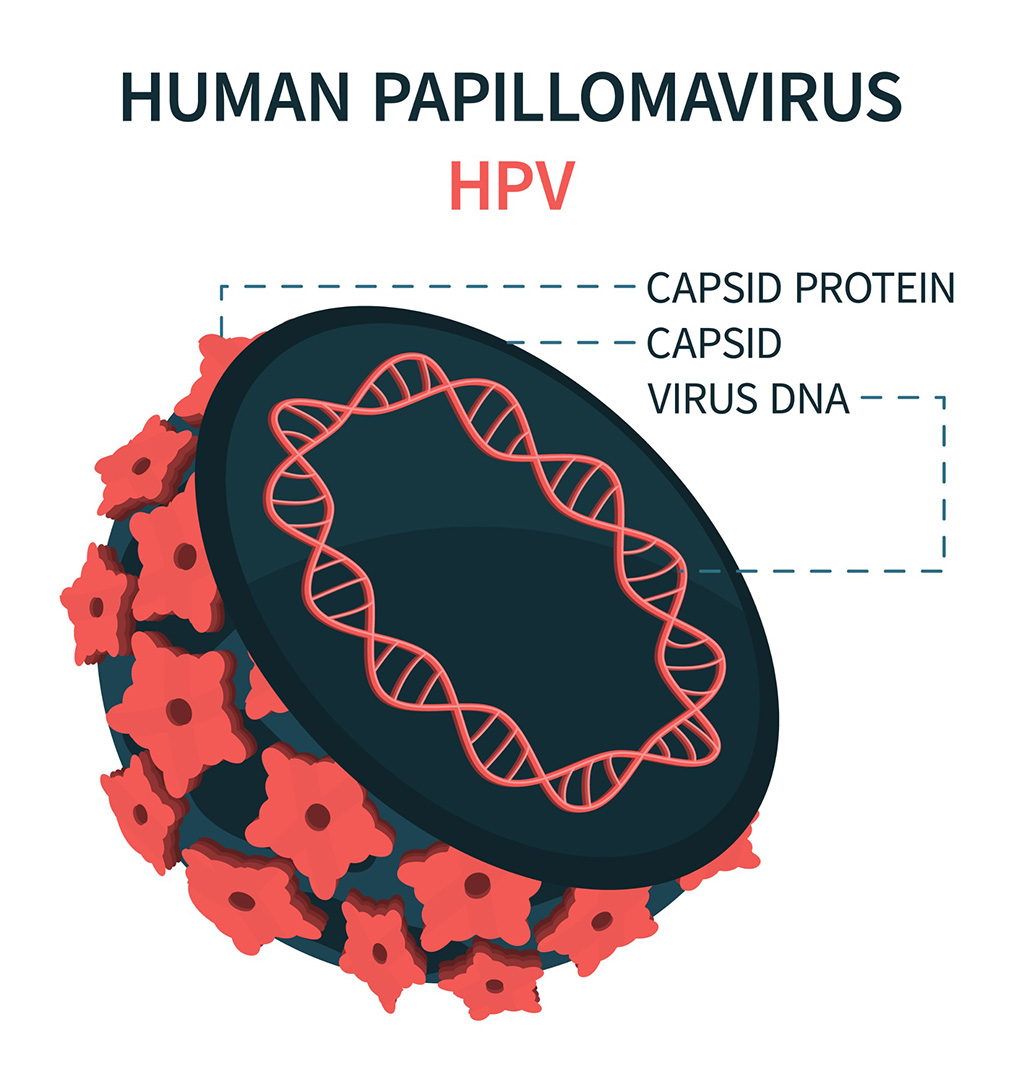
- DNA Biosensor Enables Early Diagnosis of Cervical Cancer
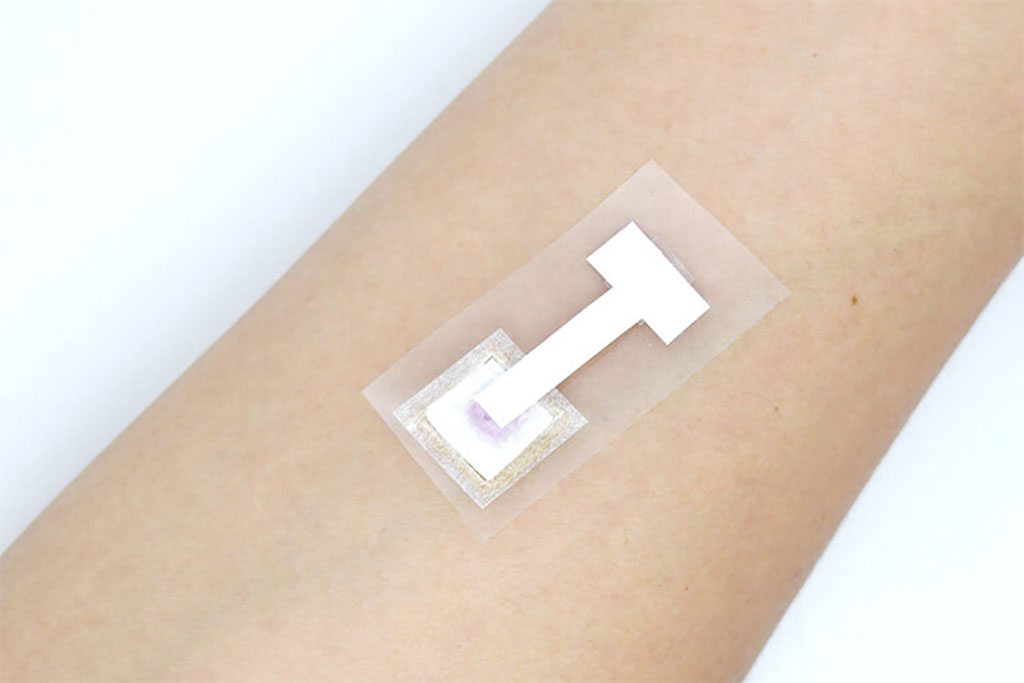
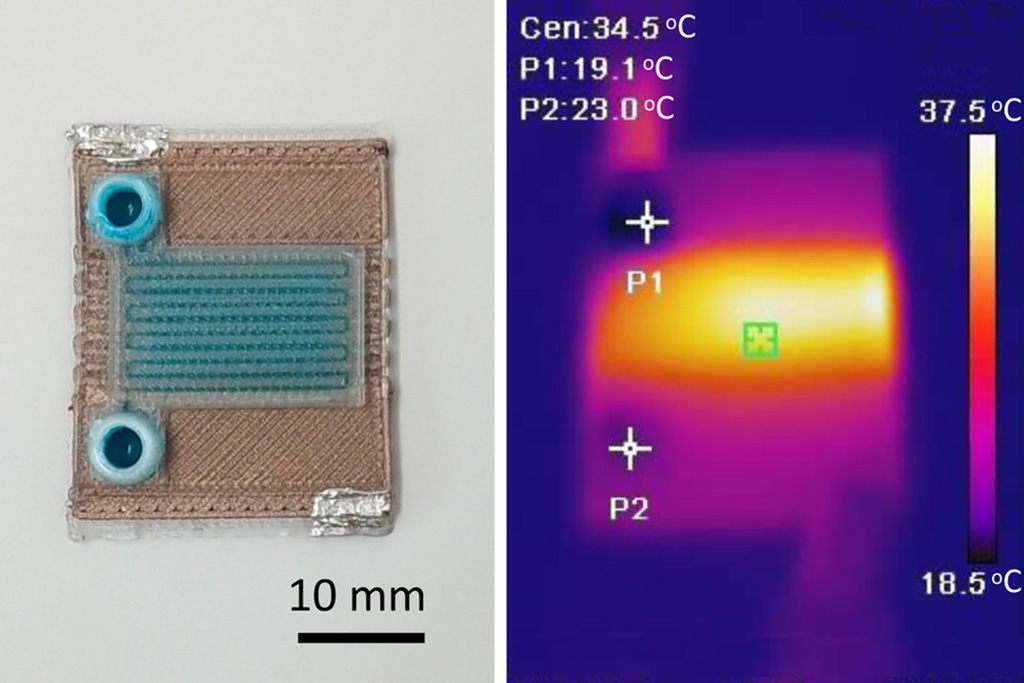
- Self-Heating Microfluidic Devices Can Detect Diseases in Tiny Blood or Fluid Samples
- Breakthrough in Diagnostic Technology Could Make On-The-Spot Testing Widely Accessible
- First of Its Kind Technology Detects Glucose in Human Saliva
- Danaher and Johns Hopkins University Collaborate to Improve Neurological Diagnosis
- Beckman Coulter and MeMed Expand Host Immune Response Diagnostics Partnership
- Thermo Fisher and Bio-Techne Enter Into Strategic Distribution Agreement for Europe
- ECCMID Congress Name Changes to ESCMID Global
- Bosch and Randox Partner to Make Strategic Investment in Vivalytic Analysis Platform
- Gene Panel Predicts Disease Progession for Patients with B-cell Lymphoma
- New Method Simplifies Preparation of Tumor Genomic DNA Libraries
- New Tool Developed for Diagnosis of Chronic HBV Infection
- Panel of Genetic Loci Accurately Predicts Risk of Developing Gout
- Disrupted TGFB Signaling Linked to Increased Cancer-Related Bacteria
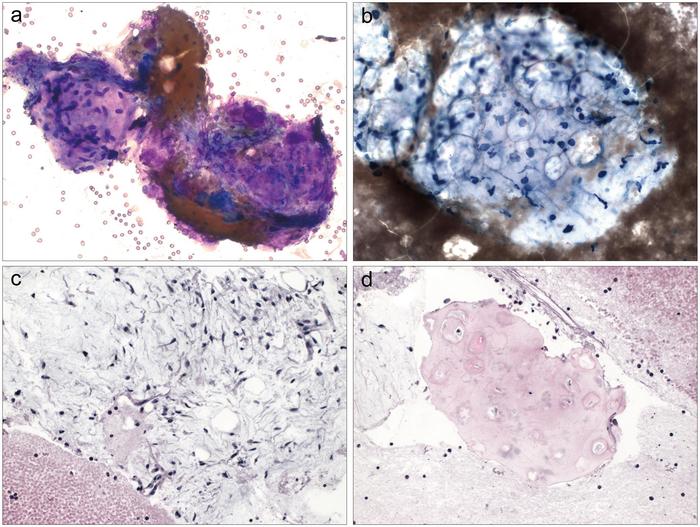
- New WHO Reporting System for Lung Cytopathology to Enhance Diagnostic Accuracy
- Self-Taught AI Tool Diagnoses and Predicts Severity of Common Lung Cancer
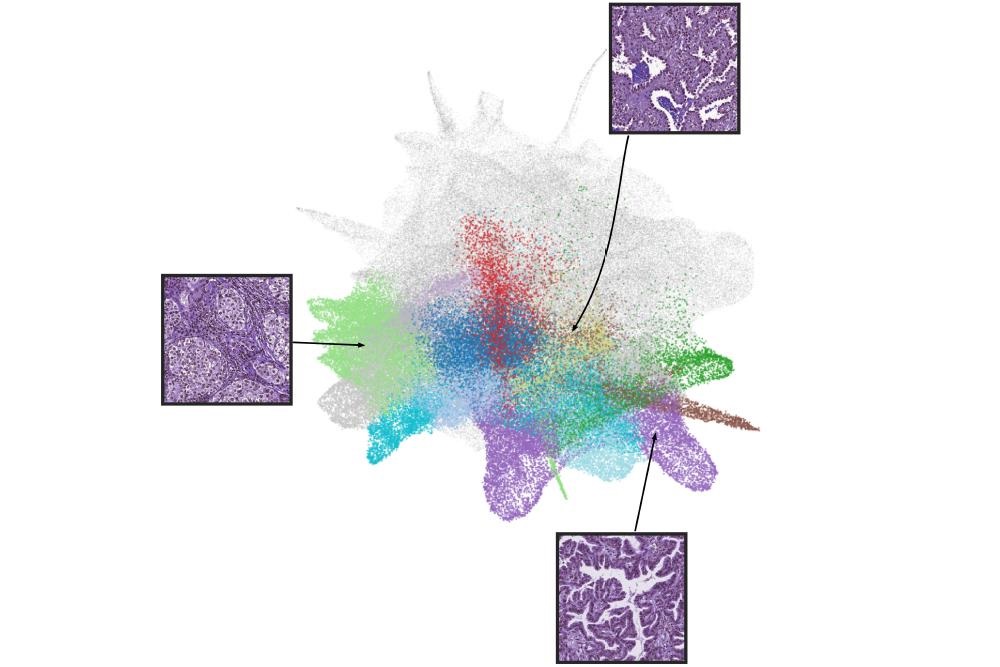
- Novel AI-Powered Method for Tissue Analysis Improves Understanding of Disease Pathology
- Noninvasive Technology Detects Rare Cancer Cells in Blood
- AI Tool Detects Tiny Protein Clumps in Microscopy Images in Real-Time

Expo
 view channel
view channel
view channel
view channel
view channel
view channel
view channel
view channel
view channel
Clinical Chem.Molecular DiagnosticsHematologyImmunologyMicrobiologyPathologyTechnologyIndustry
Events
Advertise with Us
view channel
view channel
view channel
view channel
view channel
view channel
view channel
view channel
view channel
Clinical Chem.Molecular DiagnosticsHematologyImmunologyMicrobiologyPathologyTechnologyIndustry
Events
Advertise with Us


- 3D Printed Point-Of-Care Mass Spectrometer Outperforms State-Of-The-Art Models
- POC Biomedical Test Spins Water Droplet Using Sound Waves for Cancer Detection
- Highly Reliable Cell-Based Assay Enables Accurate Diagnosis of Endocrine Diseases
- New Blood Testing Method Detects Potent Opioids in Under Three Minutes
- Wireless Hepatitis B Test Kit Completes Screening and Data Collection in One Step
- Blood-Based Test Outperforms Ultrasound in Early Liver Cancer Detection
- Four-In-One Molecular Test Detects and Differentiates Among Most Prevalent Respiratory Viruses in 20 Minutes
- First-Line PSA Testing More Cost-Effective Than First-Line MRI for Prostate Cancer Screening
- Proteomics Platform Identifies Proteins in Blood to Give Cancer Warning 7 Years before Diagnosis
- AI Technology-Based Blood Test Identifies Lung Cancer Earlier
- First 4-in-1 Nucleic Acid Test for Arbovirus Screening to Reduce Risk of Transfusion-Transmitted Infections
- POC Finger-Prick Blood Test Determines Risk of Neutropenic Sepsis in Patients Undergoing Chemotherapy
- First Affordable and Rapid Test for Beta Thalassemia Demonstrates 99% Diagnostic Accuracy
- Handheld White Blood Cell Tracker to Enable Rapid Testing For Infections
- Smart Palm-size Optofluidic Hematology Analyzer Enables POCT of Patients’ Blood Cells
- Molecular Profiling Improves Diagnosis for Children with High Risk Cancers
- Blood Test Measures Immune Response to Epstein-Barr Virus in MS Patients
- AI Predicts Tumor-Killing Cells with High Accuracy
- Diagnostic Blood Test for Cellular Rejection after Organ Transplant Could Replace Surgical Biopsies
- AI Tool Precisely Matches Cancer Drugs to Patients Using Information from Each Tumor Cell
- POC STI Test Shortens Time from ED Arrival to Test Results
- Integrated Solution Ushers New Era of Automated Tuberculosis Testing
- Automated Sepsis Test System Enables Rapid Diagnosis for Patients with Severe Bloodstream Infections
- Enhanced Rapid Syndromic Molecular Diagnostic Solution Detects Broad Range of Infectious Diseases
- Clinical Decision Support Software a Game-Changer in Antimicrobial Resistance Battle
- New Diagnostic System Achieves PCR Testing Accuracy
- DNA Biosensor Enables Early Diagnosis of Cervical Cancer
- Self-Heating Microfluidic Devices Can Detect Diseases in Tiny Blood or Fluid Samples
- Breakthrough in Diagnostic Technology Could Make On-The-Spot Testing Widely Accessible
- First of Its Kind Technology Detects Glucose in Human Saliva
- Danaher and Johns Hopkins University Collaborate to Improve Neurological Diagnosis
- Beckman Coulter and MeMed Expand Host Immune Response Diagnostics Partnership
- Thermo Fisher and Bio-Techne Enter Into Strategic Distribution Agreement for Europe
- ECCMID Congress Name Changes to ESCMID Global
- Bosch and Randox Partner to Make Strategic Investment in Vivalytic Analysis Platform
- Gene Panel Predicts Disease Progession for Patients with B-cell Lymphoma
- New Method Simplifies Preparation of Tumor Genomic DNA Libraries
- New Tool Developed for Diagnosis of Chronic HBV Infection
- Panel of Genetic Loci Accurately Predicts Risk of Developing Gout
- Disrupted TGFB Signaling Linked to Increased Cancer-Related Bacteria
- New WHO Reporting System for Lung Cytopathology to Enhance Diagnostic Accuracy
- Self-Taught AI Tool Diagnoses and Predicts Severity of Common Lung Cancer
- Novel AI-Powered Method for Tissue Analysis Improves Understanding of Disease Pathology
- Noninvasive Technology Detects Rare Cancer Cells in Blood
- AI Tool Detects Tiny Protein Clumps in Microscopy Images in Real-Time

















.jpg)



.jpeg)



.jpg)


.jpg)








_1.jpg)




