
New Malaria Test is 12 Times Faster
Malaria continues to pose a major global health threat, with approximately 247 million cases and over 600,000 deaths annually, predominantly in SubSaharan Africa. Particularly alarming is cerebral malaria, a severe form of the disease, which has
Cont’d on page 6
Latest Research Advances Blood Tests for Psychiatric and Neurological Disorders
The lack of non-invasive methods for monitoring brain status is a significant challenge in psychiatric care. Using genetic material from human blood and lab-grown brain cells, researchers have now made advances in developing a blood test to detect
brain-related changes associated with postpartum depression and other psychiatric and neurological disorders.
The research by investigators at Johns Hopkins Medicine (Baltimore, MD, USA; www.hopkins medicine.org) focused on tracing
Cont’d on page 2

See article on page 12

Test Strip Offers PCR-Level Accuracy
DAccurate Assay for Endocrine Diseases
The conventional methods for measuring free cortisol, the body’s stress hormone, from blood or saliva are quite demanding and require sample processing. The most common method, therefore, involves collecting urine over several days. However, this method requires great perseverance from patients, as collecting every drop of urine over several days can be nearly
Cont’d on page 4
Blood Test Predicts Lymphoma Outcomes
CAR T, or chimeric antigen receptor T-cell therapy, has been FDA-approved for various blood cancers such as certain types of lymphoma, leukemia, and multiple myeloma. While this innovative therapy enhances a patient’s immune cells’ ability to target and eliminate cancer cells, it is often linked with significant and occasionally severe side effects. Now, a research team
Cont’d on page 10
encing symptoms had to endure lengthy in Rapid Testing
uring the onset of the pandemic, individuals experi-
around two days for the results, to confirm if they were infected with the COVID-19 virus. This process was not only inconvenient but also involved complex and costly logistics, conqueues for lab-based PCR testing and then wait
Cont’d on page 8
Method Detects Pathogens in Blood Faster and More Accurately by Melting DNA
Globally, an alarming one in every five deaths is attributed to complications related to sepsis, with children accounting for 41% of these fatalities. Common practice involves administering antibiotics to sepsis patients while waiting for blood
culture results, which can contribute to antibiotic resistance. Ineffectively treating sepsis can be detrimental, as up to 30% of patients receive incorrect treatments, further elevating their risk of death. The critical nature of timely and accurate diagnosis in


®
14
A breakthrough blood test could replace more costly and invasive methods such as PET brain scans and cerebrospinal fluid tests in identifying Alzheimer’s indications within the brain, even before symptoms appear.
Cont’d on page
If your subscription is not renewed every 12 months your Free Subscription may be automatically discontinued Renew / Start your Free Subscription Access Interactive Digital Magazine Instant Online Product Information: Identify LinkXpress ® codes of interest as you read magazine Click on LinkXpress.com to reach reader service portal Mark code(s) of interest on LinkXpress ® inquiry matrix 1 2 3 VISIT READER SERVICE PORTAL LINKXPRESS COM ®
Alzheimer’s Blood Test Could Replace Spinal Taps and Brain Scans Alzheimer’s Blood Test Could Replace Spinal Taps and Brain Scans
® INTERNATIONAL Vol.41 No.2 • 4/2024 VISIT DAILY CLINICAL LAB NEWS
GLOBETECH MEDIA >>> <<< PUBLISHED IN COOPERATION WITH International Federation of Clinical Chemistry and Laboratory Medicine INSIDE LabMedica EXPO . 6-20 Clinical News . . . . 2-20 IFCC News .... 17-20 Industry News . .. . 21 Events Calendar . . 22 WORLD’ S CLINICA L LABORATOR Y NEW S LEADER
Image: Alzheimer’s brain showing abnormal concentrations of Tau protein tangles (blue) and beta-amyloid plaques (brown)(Courtesy of NIH)
ISSN 1068-1760
Latest Research Advances
Blood Tests for Psychiatric and Neurological Disorders
Cont’d from cover
brain cell-derived mRNAs in the bloodstream. These extracellular vesicles (EVs), which are tiny sacs containing genetic material, are crucial for cell communication and carry messenger RNA (mRNA) from the brain. This method allows for the detection of changes in gene activity within the brain. The team’s interest in this area grew from an earlier study that found altered EV communication in pregnant women who developed postpartum depression after childbirth. The latest study, published in the journal Molecular Psychiatry, used the human placenta as a model to identify 26 placental mRNAs, which are present in maternal blood during pregnancy and then disappear after birth. This discovery confirmed that mRNAs from specific tissues and organs, including the brain, can be detected in EVs found in circulating blood.


Through analysis of brain-specific mRNAs using the Human Protein Atlas and the GenotypeTissue Expression Project, the researchers identified mRNAs linked to various brain functions and disorders, including mood disorders, schizophrenia, epilepsy, and substance abuse. They also pinpointed 13 brain-specific mRNAs associated with postpartum depression. The study compared mRNAs from cells and EVs in a brain organoid model, finding that while the levels differ, they are correlated. This correlation suggests that it is possible to infer changes in the brain based on EV mRNA levels in the blood. The ultimate aim is to create a simple blood test to detect mRNA level changes related to mental disorders, potentially allowing for early detection of psychiatric emergencies like suicidal behavior. By identifying patients at risk of a psychiatric episode, intervention and prevention of adverse outcomes could be possible. Future research will focus on developing similar tests for conditions like autism spectrum disorder using lab-grown brain samples.
“This is very exciting, because right now, there isn’t a blood marker for disorders affecting the brain,” said Lena Smirnova, Ph.D., an assistant professor at the Johns Hopkins Bloomberg School of Public Health. “Essentially, these conditions are diagnosed by clinical interviews between patients and providers.”
Image: Researchers are making progress towards developing blood tests for psychiatric and neurological disorders (Photo courtesy of 123RF)
LabMedica International To view this issue in interactive digital magazine format visit www.LabMedica.com 2 LabMedica International April/2024
INTRODUCING ACUSERA SMART
STREAMLINE YOUR QC, THE SMART WAY

The Acusera Smart controls range is designed to fit directly onto a wide range of test systems without the need to aliquot material, streamlining the QC process by minimising human error and optimising workflow.
STREAMLINE WORKFLOW
With the ability to load the Acusera Smart QC and walk away, it optimises workflow by removing manual steps.
AUTOMATION
Automating the quality control process reduces turnaround time and increases efficiency.
MINIMISING HUMAN ERROR
The Smart QC workflow minimises the potential for human error by reducing the operator handling time.
REDUCED STORAGE
Can be conveniently stored onboard analysers with refrigerated compartments, for added convenience.
SIMPLIFIED PROCESS
Our SmartScan controls are linked to an XML file from Randox.com, where QC target values can be uploaded directly to the analyser, simplifying the onboarding process.
randoxqc.com
marketing@randox.com
Visit store.randox.com to buy directly from Randox today
CONVENIENT DESIGN
Our Smartload controls can be conveniently loaded directly into the analyser, eliminating the need to aliquot QC material.
103 LMI-4-24 LINKXPRESS COM
Accurate Assay for Endocrine Diseases

Cont’d from cover
impossible, even if the patients are hospitalized. This often leads to up to 60% variation in urine-free cortisol measurements in individual patients. Now, researchers have developed a new method for measuring cortisol levels directly from a blood sample, marking a significant advancement in the diagnosis and treatment of various diseases.
Researchers from Aarhus University (Aarhus, Denmark; www.international.au.dk) have discovered a groundbreaking method for measuring levels of free cortisol directly from a blood sample. This new method is simple and quick, requiring only a few drops of blood. This contrasts sharply with current practices, which are both cumbersome and inaccurate. Traditional tests, for instance, cannot differentiate between synthetic and natural cortisol. This limitation is problematic in patients who have been treated with synthetic cortisol, as it can lead to misdiagnoses or incorrect medication dosages. The new method addresses these issues by employing a cell-based assay, which not only improves the accuracy and reliability of cortisol measurements but also reduces the high variation commonly seen in patient samples.
The most common method currently is immunoassays using antibodies. However, the new method uses the cell-based assay HEK293F-GRE, which allows for the measure-
ment of the total level of cortisol, including both natural free cortisol and synthetic cortisol from medicinal products. This new test has the potential to be a game-changer for diagnosing and treating patients who require cortisol regulation. This includes individuals with stress-related illnesses like anxiety and depression, chronic diseases such as diabetes and cancer, and inflammatory diseases like allergies and asthma. While there is still a need to explore how to best integrate this method into clinical practice, the goal is to make the test available using a standard blood sample for doctors, thereby improving patient care.
“Being able to measure the total cortisol level accurately means we can potentially adapt treatment more precisely and reduce the risk of side effects,” said Andreas Lodberg, MD and postdoc at the Department of Biomedicine, Aarhus University. “Our validation shows that this method meets the stringent criteria set by the U.S. Food and Drug Administration, making it a promising candidate for future use in clinical laboratories.” The researchers’ findings are detailed in a study published in the journal Analytical Chemistry on January 26, 2024.
Image: The new versatile assay has the ability to measure both total and bioavailable cortisol from serum (Photo courtesy of Aarhus University)
Test Could Predict Immunotherapy Success for Broader Range Of Cancers
Immunotherapy has revolutionized cancer treatment, yet only a minority of patients experience favorable responses. Determining which patients will benefit from immunotherapy is critical, as these treatments can have severe side effects and, in some cases, may even accelerate cancer progression. Now, a new genetic marker has been identified that could predict patient response to immunotherapy in cancer types that previously lacked such predictive tools.
A study led by researchers at the University of Pittsburgh (Pittsburgh, PA, USA; www. pitt.edu) has found that tumors with a higher number of intragenic rearrangements (IGRs) in their genetic code may respond better to immunotherapy. This finding opens the door to more targeted treatment decisions for cancers like breast, ovarian, esophageal, and uterine cancer. Typically, immunotherapy responders are identified by the tumor muta-
Cont’d on page 5
Graham Beastall United Kingdom
Hernán Fares Taie Argentina
Bernard Gouget France
Maurizio Ferrari Italy
Tahir S. Pillay South Africa
Andreas Rothstein Colombia
Praveen Sharma India
Rosa I. Sierra-Amor Mexico
Peter Wilding United States
Andrew Wootton United Kingdom

4 LabMedica International April/2024 LabMedica lnternational is published eight times a year and is circuIated worldwide (outside the USA and Canada) without charge and by written request, to clinical laboratory specialists and administrators, and other qualified professionals allied to the field. To all others: Paid Subscription is available for a two-year subscription charge of US$120. Single copy price is US$20. Mail your paid subscription order accompanied with payment to Globetech Media, P.O.B. 800222, Miami, FL 33280-0222. For change of address or questions on your subscription, write to: LabMedica lnternational, Circulation Services at above address; or visit: www.LinkXpress.com SUBSCRIPTION INFORMATION Vol.41 No.2. Published, under license, by Globetech Media LLC; Copyright © 2024. All rights reserved. Reproduction in any form is forbidden without express permission. Opinions expressed are solely those of the authors, and do not represent an endorsement, or lack thereof, by the Publisher of any products or services. ISSN 1068-1760 ADVERTISING SALES OFFICES HOW TO CONTACT US labmedica.com A GLOBETECH PUBLICATION Published in cooperation with the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC). LabMedica International • LabMedica en Español • LabMedica.com HospiMedica International • HospiMedica.com • MedImaging.net HospiMedicaExpo.com • LabMedicaExpo.com • LinkXpress.com Subscriptions: Send Press Releases to: Advertising & Ad Material: Other Contacts: www.LinkXpress.com LMNews@globetech.net ads@globetech.net info@globetech.net EDITORIAL BOARD
INTERNATIONAL Teknopress Yayıncılık ve Ticaret Ltd. Şti. adına İmtiyaz Sahibi: M. Geren • Yazı işleri Müdürü: Ersin Köklü Müşir Derviş İbrahim Sok. 5/4, Esentepe, 34394 Şişli, İstanbul P. K. 1, AVPIM, 34001 İstanbul • E-mail: Teknopress@yahoo.com Baskı: Postkom A.Ş. • İpkas Sanayi Sitesi 3. Etap C Blok • 34490 Başakşehir • İstanbul Yerel süreli yayındır. Yılda sekiz kere yayınlanır, ücretsiz dagıtılır. Dan Gueron David Gueron Sanjit Dutt Carolyn Moody, RN Simone Ciolek Parker Xu Karina Tornatore Publisher Managing Editor News Editor Regional Director Regional Director Regional Director Reader Service Manager USA Miami, FL 33280, USA Carolyn.Moody@globetech.net Tel: (1) 954-686-0838 GERMANY, SWITZ., AUSTRIA Bad Neustadt, Germany Simone.Ciolek@globetech.net Tel: (49) 9771-1779-007 OTHER EUROPE & UK Miami, FL 33280, USA Carolyn.Moody@globetech.net Tel: (1) 954-686-0838 JAPAN Tokyo, Japan Katsuhiro.Ishii@globetech.net Tel: (81) 3-5691-3335 CHINA Shenzen, Guangdong, China Parker.Xu@globetech.net Tel: (86) 755-8375-3877 OTHER COUNTRIES Contact USA Office ads@globetech.net Tel: (1) 954-686-0838 Founder & Editorial Director Marc Gueron
LabMedica International To view this issue in interactive digital magazine format visit www.LabMedica.com
Test Could Predict Immunotherapy Success for Broader Range of Cancers
Cont’d from page 4
tional burden (TMB), which counts the number of simple mutations in cancer cells' DNA. High TMB makes cancer cells more conspicuous to the immune system, making patients with high TMB ideal candidates for immunotherapy. However, this method is more effective for cancers with high mutation rates, such as melanoma and lung cancer.
Searching for a new genetic biomarker to predict the team analyzed over 1,000 cancer genomes from various types using a large database from the International Cancer Genomic Consortium. They found that assessing the overall IGR burden could predict immune cell infiltration and immunotherapy response in certain cancers. IGRs, resulting from complex structural rearrangements within a gene, contrast with TMB, which is similar to simple typos in DNA. The researchers observed a relationship between IGR and TMB across different tumor types. Cancers with high TMB, like melanoma and lung cancer, had low IGR burdens, while breast, ovarian, endometrial, and esophageal cancers exhibited low TMB but high IGR burdens. Both high IGR and high TMB were associated with increased T cell inflammation, suggesting that either type of genetic abnormality could enhance immune recognition and potential destruction of cancer cells.
In a clinical trial of esophageal cancer patients treated with durvalumab, those with higher IGR burdens responded better to the immunotherapy, while relapsed patients tended to have lower IGR burdens. Another clinical trial with bladder cancer patients treated with atezolizumab showed that TMB, but not IGR burden, predicted response in this TMB-dominant cancer. However, in patients pre-treated with platinum-based chemotherapy, post-chemotherapy IGR burden, but not pre-chemotherapy burden, predicted immunotherapy response. This suggests that platinum exposure, which can induce structural genome rearrangements, affects immunotherapy response. These findings suggest that IGR testing could guide clinicians in selecting optimal treatments for different cancer types and contexts. With the declining cost of whole genome sequencing, measuring IGR burden could soon be both feasible and affordable. The researchers are now focusing on further developing and standardizing their tests and algorithms for clinical application.
“Genetic tests are available that are great at predicting immunotherapy response in melanoma and lung cancer, but they don’t work well in some other cancers,” said Xiaosong (Johnathan) Wang, M.D., Ph.D., associate professor of pathology at Pitt. “To help fill this gap, we developed a new approach, which analyzes a class of hidden, or cryptic, rearrangements in the cancer genome.” The findings were published in the journal Cancer Immunology Research.

• Elevated Lp(a) is a risk factor, even at very low LDL-C concentration
• Lp(a) level is determined by genetics, which should be measured once in adults
• Lp(a) 21 FS provides measurement in molar concentration (nmol/L) or mass (mg/dL)
• Isoform-insensitive assay with harmonized calibrators and controls
• Standardized to international WHO-IFCC reference material SRM 2B
DiaSys. Total Confidence in Patient Results. www.diasys-diagnostics.com

5 LabMedica International April/2024 105 LMI-4-24 LINKXPRESS COM LabMedica International To view this issue in interactive digital magazine format visit www.LabMedica.com
Image: Correctly identifying which patients will respond to immunotherapy and those who will not is crucial (Photo courtesy of 123RF)

CYSTIC FIBROSIS TEST DIASORIN MOLECULAR

xTAG Cystic Fibrosis (CFTR) is a multiplexed nucleic acid test intended for the detection of clinically relevant mutations by ACMG/ACOG, offering clinicians the ability to test for the 23 CFTR mutations recommended by ACMG/ACOG.
202 LMI-4-24 COM
Tuberculosis Test to Expand Testing Access in Emerging Countries
Tuberculosis (TB) is one of the top infectious killers worldwide, second only to COVID-19 in recent years. Keeping track of how well treatment works is key to fighting this disease. However, testing TB samples is tricky because it’s highly infectious, requiring special high-security labs known as biosafety level 3 (BSL-3) labs. These labs have special equipment to keep the virus from spreading, but they’re expensive and hard to find, especially in low- and middle-income countries where most TB cases are found. This makes fighting TB difficult in the places that need it most. Now, a new test allows testing for TB treatment monitoring to be done outside of a BSL-3 laboratory, potentially making it easier and faster to treat TB worldwide.
The new TB test – called rapid enumeration and diagnostic for tuberculosis (READ-TB) – was disclosed in the Association for Diagnostics & Laboratory Medicine’s (ADLM, Washington, DC, USA; www.myadlm. org) Clinical Chemistry journal and has been designed specifically to address the lack of access to labs that meet BSL-3 requirements. The test works by treating sputum (lung mucus coughed up by patients) with acetic acid. This acid kills the TB bacteria but leaves their RNA (genetic material) untouched. A lab worker can then safely check the RNA levels with a machine, which tells how much TB bacteria is in the patient and how well the treatment is working.
Acetic acid is better at preserving RNA than the current gold standard of using guanidium salts for two reasons: it kills the bacteria in just 30 minutes, thus eliminating the need for a BSL-3 lab, and it’s just as effective at keeping the RNA safe. The RNA remains stable for 14 days at room temperature and for over a year if frozen at -20°C. This is ideal for labs without the capacity for super-cold storage. Plus,
Cont’d from cover
DIABETES CONTROL RANDOX LABORATORIES

203 LMI-4-24 LINKXPRESS COM To

The Acusera HbA1c is a lyophilized control with assayed values provided for HPLC and a range of chemistry analyzers. It provides 100% human whole blood control, which helps minimize matrix effects reducing lot-to-lot variations.

acetic acid is less toxic than guanidium salts, making it safer for lab workers. The READ-TB test isn’t just safer and easier; it could also help with TB drug research. By accurately measuring TB RNA levels, scientists can compare different TB drugs or drug combinations more effectively.
“We wanted to improve the assay for integration into diagnostic microbiology laboratories, for use in clinical trials, and to make it tenable in low- and middle-income countries, e.g., where no BSL-3 laboratory exists,” the authors of the study write, referring to their earlier test. “READ-TB allows measurement of the molecular bacterial load to now be adopted by routine clinical microbiology laboratories for measuring M. tuberculosis bacterial load in sputum.”
New Malaria Test is 12 Times Faster
a high mortality rate, especially among children under five. The existing rapid diagnostic tests (RDTs) for malaria offer a basic positive or negative result, but often fail to detect asymptomatic infections and lack the sensitivity required for early detection of severe cases. More sensitive molecular assays that exist are costly, time-consuming, and need specialized skills and equipment, rendering them unsuitable for widespread application in areas with limited resources. In light of these challenges, researchers have now developed a new test for diagnosing malaria that is both rapid and accurate. This POC rapid malaria test offers a significant improvement over traditional tests and will be especially beneficial for rural areas with limited healthcare facilities.
Cont’d on page 8
6 LabMedica International April/2024
receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
Image: The new tuberculosis test could improve TB care globally (Photo courtesy of Shutterstock)
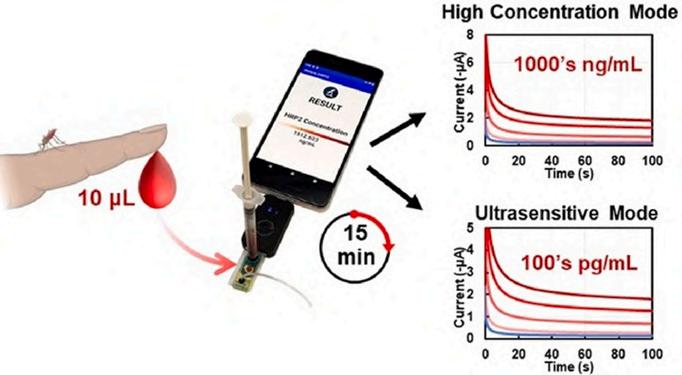
Image: Researchers have developed a faster and simpler point-of-care malaria test (Photo courtesy of Rice University)


107 LMI-4-24 LINKXPRESS COM

Cont’d from cover


Test Strip Offers PCR-Level Accuracy in Rapid Testing
tributing to testing delays and increasing the risk of spreading the disease. Now, a newly developed biosensing technology enables the creation of gene test strips that can match the quality of traditional lab-based tests.
The new technology developed by a team of biomedical engineers at UNSW Sydney (Sydney, Australia; www.unsw.edu.au) offers test strips that are as accurate as lab-based PCR tests, with the added advantage of quick, on-site disease detection. Described by the researchers as having “PCR in your pocket,” this advancement holds potential for broad applications in biomedical and environmental diagnostics across various sectors, including food, agriculture, and biosafety management. The technology allows for the detection of specific gene sequences at room temperature, using test strips that resemble the familiar RAT Covid test, potentially eliminating the need for long queues at PCR testing centers and drastically reducing costs to a few dollars per test. The test strips could be instrumental
in rapidly responding to new pathogens, identifying areas with high antibiotic resistance, or in conservation efforts for endangered species.
The process of achieving PCR-level accuracy with these new test strips involves the creation of minuscule DNA nano-circles, each containing a fragment of the target DNA, such as the COVID virus. These nano-circles, approximately 2 nanometres in size, are then combined with CRISPR/Cas proteins, which are programmed to interact specifically with the target pathogen’s DNA. When these proteins encounter the target DNA, they cause the DNA nano-circles to linearize, creating an abundance of ‘fake targets.’ This method triggers a molecular chain reaction, resulting in a flood of these fake targets that are easily detectable by the test strips, even with minimal presence of the original gene target.
This technology has been demonstrated to accurately detect COVID-19 virus and Helicobacter bacteria, which is responsible for stomach ulcers. Potential applications of

this biosensing method extend beyond health diagnostics to include biosecurity (detecting invasive marine species), environmental science (tracking threatened species through DNA testing of environmental samples), and even cancer diagnosis, as demonstrated by the team’s successful detection of cancer mutations in clinical patient samples.
“We think we created a new benchmark in biosensing – our gene-based tests will be able to be performed anywhere, anytime, by virtually anyone,” said study author Dr. Fei Deng. The study was published in Nature Communications on March 5, 2024.
New Malaria Test is 12 Times Faster
Cont’d from page 6
Researchers at Rice University (Houston, TX, USA; www.rice.edu) developed a microfluidic point-of-care (mPOC) immunoassay for quantifying a malaria parasite biomarker, Plasmodium falciparum histidine-rich protein 2 (PfHRP2), in whole blood. This device provides dual diagnostic modes to detect PfHRP2 at low and high concentrations, making it versatile for various diagnostic needs, such as identifying asymptomatic infections and predicting disease progression. The test produces results in just 15 minutes, which can be easily accessed via a smartphone app developed by the researchers. Field tests in Malawi revealed that the mPOC
immunoassay matches the accuracy of the standard PfHRP2 enzyme-linked immunosorbent assay (ELISA), but is 12 times quicker and simpler to operate. This advancement in malaria diagnostics, especially for cerebral malaria, holds the promise of early detection and prompt treatment of severe cases, potentially saving numerous lives.
“The mPOC immunoassay was designed to be simple, accurate and field-deployable, making it suitable for use in rural and remote health centers in sub-Saharan Africa,” said mechanical engineer Peter Lillehoj of Rice’s Brown School of Engineering who led the research team. “Unlike traditional tests, this
device does not require plasma separation, pipetting, complicated sample processing or long incubations, making it easy to use even by minimally trained health care providers.”
“In areas with limited access to health care facilities, our test could be a game-changer,” added Lillehoj. “It can help health care providers quickly identify and treat severe cases, potentially saving lives. By enabling early detection and appropriate management of malaria cases, we can reduce the burden of the disease and improve patient outcomes in Africa and beyond.” The team published their work on February 6, 2024 in the journal Biosensors and Bioelectronics.
8 LabMedica International April/2024 IMMUNOASSAY QC MATERIAL LGC CLINICAL DIAGNOSTICS
IA Plus Control is intended for use as a third-party, multi-constituent quality control material to monitor the precision of laboratory testing procedures for Immunoassay
TUBE ROTATOR GLOBE SCIENTIFIC
GTR-HA tube rotator provides slow to vigorous mixing of biological samples and solutions. The unit includes a rotisserie-style stainless steel plate and interchangeable tube holder clips for 1.5mL, 15mL & 50mL tubes. 205 LMI-4-24 COM 206 LMI-4-24 LINKXPRESS COM To receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
Multichem
ANALOG
The
Image: New test strips raise the game in genebased diagnostics (Photo courtesy of 123RF)
Cell Sorter Chip Technology to Pave Way for Immune Profiling at POC
Monitoring the response of the immune system of cancer patients during disease and treatment is important for achieving favorable outcomes. To do this, labs utilize flow cytometry to perform immune profiling, which involves identifying and quantifying a patient’s immune cells at a specific time. This information is vital for determining the most effective treatment for a cancer patient. Continuing this profiling during treatment helps clinicians understand how well the treatment is working. Despite the promise of immune profiling in guiding therapy being a burgeoning area in cancer research and treatment, it hasn’t been widely adopted in clinical practice due to the high cost, large size, and complexity of flow cytometry equipment. These machines are confined to specialized labs, and transporting blood samples to these facilities is time-consuming and requires special conditions to keep the cells viable, making routine monitoring of cancer patients challenging. Now, a novel cell sorter chip technology could bring immune monitoring to clinical practice for creating a truly personalized cancer treatment plan.
Researchers at IMEC (Leuven, Belgium; www.imec-int.com) have developed a cytometry and cell sorter technology that channels a sample with fluorescently labeled cells through microfluidic channels on a chip. The cells are guided towards an excitation laser, a detection unit, and a sorting junction, where target cells are directed into a side channel using vapor bubbles created by microheaters in a water-filled microchamber. The target cells can then be collected and quantified at the end of this fluidic side channel. This technology could allow oncologists to use a portable tool, about the size of a lunchbox, in their office or daycare center to periodically check a patient’s immune system.
The process would involve taking a drop of blood and inserting the chip cartridge into an analyzing desk top tool, with results available within minutes. The chip, produced using standard chip technology on 200mm silicon wafers, can be mass-produced at an affordable cost, making it dispos able if necessary to avoid cross-sample contamination. Additionally, the fabri cation process allows for multiple mi crofluidic channels per chip, enabling high throughput without sacrificing sensitivity. This could mean a complete immune signature in as little as 10 minutes. By comparing these immune signatures to larger datasets, oncologists can quickly determine if a therapeutic effect is achieved.

flow cytometry equipment. They chose to study CD8+PD-1+ T-cells, a type of cell that displays both CD8 and PD-1 proteins on its surface. CD8 signifies the cells as cytotoxic T-cells, essential for recognizing and destroying targets like cancer cells. PD-1 is a protein that regu-

Global IVD Manufacturer dream come true

In the first clinical validation of the cell sorter chip technology, IMEC researchers along with their colleagues at KU Leuven (Leuven, Belgium; www.kuleuven.be) set out to investigate whether it could identify and quantify immune cells as effectively as

109 LMI-4-24 LINKXPRESS COM 9 LabMedica International April/2024
LabMedica International To view this issue in interactive digital magazine format visit www.LabMedica.com
With OWN BRAND Chemiluminescence Immunoassay system IncreCare is a manufacturer focusing on R&D and Manufacturing of Medical equipment/IVD instrument
Open chemiluminescence Immunoassay platform, Full-throughput, 5000+ installation in 3 years 600T/h 900T/h Shine i8000/9000 300T/h Shenzhen Increcare Biotech Co., Ltd W: www.increcare.com E: marketing@increcare.com A: 12th Floor, Building 5, Namtai INNO Park, No. X259 Guangming Avenue, Guangming District, Shenzhen, Guangdong, China T: 0086-0755-29182212 www.increcare.com
Cont’d on page 10
Image: Cytometry set-up with IMEC’s cell sorter chip (Photo courtesy of IMEC)




Blood Test Predicts Lymphoma Outcomes
Cont’d from cover
has discovered that two routine and easily conducted blood tests can predict which patients face a higher risk of adverse outcomes following treatment with CD19-targeted CAR T cells. This finding offers a chance to enhance the safety and effectiveness of this rapidly evolving category of cancer immunotherapies.
The study by a team of collaborators from Roswell Park Comprehensive Cancer Center (Buffalo, NY, USA; www.roswellpark.org) and Moffitt Cancer Center (Tampa, FL, USA; www.moffitt.org) involved 146 patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL). These patients, having previously undergone at least two prior lymphoma therapies, were treated with the CAR T immunotherapy axicabtagene ciloleucel (also known as axi-cel, and marketed as Yescarta). A significant majority of these patients (93%) experienced cytokine release syndrome (CRS) post-CAR T treatment, and over half (61%) developed immune effector cell-associated neurotoxicity syndrome (ICANS).
The researchers identified that patients with baseline serum blood levels of c-reactive protein (CRP) at or above 4 mg/dL and ferritin levels of 400 ng/mL or higher are at the greatest risk of poor outcomes. This includes reduced progression-free and overall survival, as well as elevated rates of severe toxicities. While numerous studies have focused on characterizing and assessing the effectiveness of CAR T therapy post-treatment, there was previously no readily accessible lab test or biomarker to quickly pinpoint patients at high risk for adverse outcomes before receiving CAR T-cell infusion.
“We determined that two common and easily measured blood tests can identify in advance which patients are at high risk for poor outcomes after treatment with CD19-targeted CAR T cells,” said Marco Davila, MD, PhD, Senior Vice President and Associate Director for Translational Research at Roswell Park. “We’re excited because these findings not only help us to make CAR T-cell therapies work for more patients with hard-to-treat cancers, they also help us spare some patients from additional medications they don’t need.”
“Despite encouraging outcomes with CAR T-cell therapy for hardto-treat B-cell lymphoma, some patients experience toxicity and poor outcomes. Our work determined that readily available lab tests for c-reactive protein and ferritin can identify which patients, pre-treatment, are at high risk for side effects and not responding to CD19-targeted CAR T-cell therapy,” added Rawan Faramand, MD, Assistant Member of the Blood and Marrow Transplant and Cellular Immunotherapy Department at Moffitt Cancer Center. The researchers published their findings in Blood Cancer Discovery on March 1, 2024.

Cell Sorter Chip Technology to Pave Way for Immune Profiling at POC
Cont’d from page 9
lates T-cell activity, maintaining immune balance after an invader is attacked. However, cancer cells can manipulate this pathway by overexpressing PD-1 ligands, suppressing the immune response, and allowing unchecked growth. Analyzing PD-1 positive T-cells in a cancer patient’s blood can indicate whether the cancer employs this immune evasion strategy.
In such cases, immune checkpoint inhibitor drugs can be used to prevent PD-1 proteins on T-cells from binding with tumor-produced PD-1 ligands, restoring the T-cells’ ability to attack the cancer cells. The chip accurately identified PD-1 positive T-cells in blood samples from 15 ovarian cancer patients, matching the accuracy of conventional, expensive flow cytometry equipment like FACS. This achievement marks a significant step towards developing a point-of-care tool for immune profiling, a groundbreaking advancement in cancer therapy that aims to customize treatment plans for each patient.
“With cancer, you don’t want to lose precious time by giving the patient an expensive therapy that doesn’t work. This is certainly true for ovarian cancer. This kind of cancer is mostly detected at a very late stage because the tumor has a lot of ‘free’ space to grow in the abdominal cavity before the patient will experience this as pain,” said An Coosemans, MD Ph.D., professor at KU Leuven and heading the Laboratory on Tumor Immunology and Immunotherapy. “Imec’s cell sorter technology has the potential to provide oncologists with a tool to do initial and repeated immune profiling to choose the most effective treatment.”
10 LabMedica International April/2024 FLUORESCENCE MICROSCOPE HUMAN GMBH
HumaScopeFluo LED fluorescence microscope uses mercury-free and WHO-recommended LED fluorescence technology. It is based on the 2-channel fluorescence illumination technique and does not require bulb replacement. BENCHTOP CHEMILUMINESCENCE ANALYZER LABTEST
Vercentra CS-3000 is a benchtop chemiluminescence analyzer with a throughput of up to 240 tests/ hour and features a washing station with a two-step cleaning system, consisting of six needles to ensure high efficiency. 208 LMI-4-24 COM 209 LMI-4-24 LINKXPRESS COM To receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
The
The
Image: Baseline serum inflammatory proteins predict poor CAR T outcomes in diffuse large B-cell lymphoma (Photo courtesy of 123RF)
Wireless Hepatitis B Test Kit Completes
Screening and Data Collection in One Step
Hepatitis B, a significant global health concern, is responsible for chronic liver diseases like cirrhosis and liver cancer which is one of the most common cancers worldwide. The challenge with hepatitis B is that most carriers are asymptomatic, remaining unaware of their infection, which can lead to its unintentional spread. Thus, early diagnosis is vital to halt the spread, initiate timely treatment, and reduce the risk of liver complications primarily caused by the hepatitis B virus. However, conventional tests for hepatitis B surface antigen (HBsAg) and hepatitis B e antigen (HBeAg) are complex, requiring specialized skills and large, expensive machine-based assays typically found in major hospitals. To overcome the problem of inadequate access to screening for Hepatitis B, researchers have developed a wireless, pointof-care test for the Hepatitis B Virus that can make diagnosis simpler and faster.
Researchers from Chulalongkorn University (Bangkok, Thailand; www.chula.ac.th) have developed a testing kit that enables healthcare providers in smaller medical facilities or public health centers to independently conduct hepatitis B screenings. This approach simplifies the screening process, yielding results comparable to those of sophisticated machine-based tests. The kit utilizes an electrochemical biosensor based on the specific interaction between antigens and antibodies. The presence of the hepatitis B virus triggers a detectable change in electric current through amperometric detec-
Htion. This change in current, correlating with the virus's presence, offers a semi-quantitative measurement of the antigen's concentration.
The testing process is quick and requires only a small serum volume (2 µl) from a blood sample for application and incubation on the electrodes. Following a wash with a buffer solution and drying, it takes merely up to 10 minutes to observe the resultant electrical current changes. Unlike traditional tests that merely indicate the presence or absence of the antigen, this wireless test not only con firms infection but also provides an estimated viral load. A low current suggests a higher antigen quantity, and vice versa. In addition to delivering rapid results with an approximate viral count, the innovative test also enables immediate, real-time data upload, linking the information directly to the respective patient. This feature is particularly crucial for hepatitis B, where patient identification is essential due to the prolonged nature of the treatment.
“In the early years, we developed a pro totype of a hepatitis B virus test kit using an electrochemical biosensors technique that works with a variety of measuring instru ments,” said Dr. Natthaya Chuaypen from Chulalongkorn University. “In the following year, for the kit to have reproducibility, and stability of current, with fast and convenient use, we developed the test kit with Bluetooth capability, so it can work both wirelessly and plugged into a small computer. We’re now in the process of collecting data for field visits,
Simple Blood Test Can Predict Heart Attack Risk within 6 Months
eart attacks are the leading cause of death globally, with their incidence on the rise. Despite this, many high-risk individuals either remain unidentified or fail to adhere to preventive treatments. Notably, the period preceding a heart attack is marked by significant biological changes. For instance, the risk of a heart attack doubles in the month following a divorce and increases fivefold in the week after a cancer diagnosis. Based on the hypothesis that several vital biological processes are active during the months before a heart attack, researchers have now suggested that these could be detected using a simple blood test. Researchers at Uppsala University (Uppsala, Sweden; www.uu.se) have developed an online tool that, when used in conjunction with standard blood test results, can help clinicians determine if a person is at an elevated risk of experiencing a heart attack within the next six months. The study involved analyzing blood samples from 169,053 individuals from six European cohorts who had no previous history of cardiovascular disease. Of these participants, 420 suffered their first heart attack within six
months. Their blood samples were then compared with those from 1,598 healthy cohort members.
The research team identified approximately 90 molecules associated with an increased risk of a first heart attack. Interestingly, existing healthcare blood samples are sufficient to predict this risk. The research team plans to further explore these 90 newly identified molecules to better understand them and investigate potential treatment opportunities. Additionally, the online tool created by the researchers enables individuals to assess their sixmonth heart attack risk. This tool is intended to boost patients' motivation to adopt healthier lifestyles, such as adhering to preventive medication regimens or quitting smoking.


“We wanted to develop methods that would enable the health services to identify people who will soon suffer their first heart attack,” said Professor Johan Sundström at Uppsala University who led the research team. The team’s findings were published in the journal Nature Cardiovascular Research on February 12, 2024

LabMedica International To view this issue in interactive digital magazine format visit www.LabMedica.com 11 LabMedica International April/2024
Image: Wireless Point-of-Care Testing for Hepatitis B Virus (Photo courtesy of Chulalongkorn University)
+86 592 6807188 intecproducts@asintec.com www.intecasi.com ABO & RhD BLOOD GROUPING RAPID TEST Exclusive patented fully solid-phase technology
result interpretation 1 Drop 1 Minute Know Your Blood Type within One Minute Built-in quality control dots Clear and easily readable results, eliminating subjective errors Ctl Easy and safe operation Room temperature storage
Standardized
111 LMI-4-24 LINKXPRESS COM

To

The GH-3100 is a 3-part hematology analyzer featuring advanced technology for accurate differentiation between neutrophils, lymphocytes, and monocytes and is capable of processing 60 samples per hour with just 9 μL of blood.
Alzheimer’s Blood Test Could Replace Spinal Taps and Brain Scans
Historically, Alzheimer’s disease was primarily diagnosed based on observable symptoms, particularly when individuals started exhibiting memory and cognitive difficulties. However, it’s been revealed through research that up to a third of individuals diagnosed with Alzheimer’s based solely on cognitive symptoms have been incorrectly diagnosed, with their symptoms stemming from other causes. The accurate identification of Alzheimer’s disease has become increasingly crucial, especially since the introduction of the first treatments that can slow the disease’s progression, along with other promising drugs currently in development. These treatments are potentially more effective when administered early, highlighting the need for early detection of the disease. Therefore, to qualify for Alzheimer’s therapies, patients must show cognitive impairment and test positive for amyloid plaques, which are distinctive to Alzheimer’s. Techniques like amyloid positron emission tomography (PET) brain scans, cerebrospinal fluid analyses, and blood tests are used to detect brain amyloid plaques. However, these are only employed for individuals already showing cognitive symptoms and not for asymptomatic individuals.
Now, a study by researchers at Washington University School of Medicine in St. Louis (WUSM, St. Louis, MO, USA; www.wustl.edu) and Lund University (Lund, Sweden; www.lunduniversity.lu.se) has demonstrated that a blood test can diagnose Alzheimer’s disease pathology as accurately as cerebrospinal fluid tests and brain scans. This is true even for patients with mild symptoms and can detect molecular signs of Alzheimer’s in the brain before symptoms appear. Developed by researchers at Washington University, this blood test employs a highly sensitive technique to measure Alzheimer’s protein levels in the blood. It works by using mass spectrometry to assess the ratio of two amyloid forms in the blood and was granted Breakthrough Device designation by the FDA in 2019.
Following this, the researchers have introduced a second blood test focusing on the impact of amyloid accumulation on another brain protein, tau. Amyloid presence in the brain alters the levels of various tau protein forms both in the brain and the blood. The ratio of phosphorylated tau-217 (ptau-217) to unphosphorylated tau in the blood is a reliable indicator of brain amyloid levels. In their latest study, the researchers compared four different tests for their ability to detect amyloid in the brain: the ptau-217 blood test and three FDA-approved cerebrospinal fluid tests. They assessed these tests using blood and cerebrospinal fluid samples from two groups of volunteers - one cohort of 1,422 people and a second cohort of 337 people, including individuals
Humasis Malaria Test is one step in vitro diagnostic test based on immunochromatographic assay. It is designed for detection of P. falciparum (HRPII) and pLDH(P. falciparum, P. vivax, P. ovale, P. malariae) in human blood. 211



with very mild and mild cognitive symptoms, as well as healthy individuals for comparison. The accuracy of these tests was determined by comparing their results with the gold standard of PET brain scans for amyloid and tau tangles.
The findings showed that the ptau-217 blood test matched the FDAapproved cerebrospinal fluid tests in accurately identifying individuals with amyloid buildup, with all tests achieving accuracy rates between 95% and 97%. In a further analysis focusing on the detection of tau tangles in the brain, the ptau-217 blood test outperformed cerebrospinal fluid tests, achieving accuracy rates ranging from 95% to 98%. An additional analysis focusing on healthy participants revealed that the ptau217 blood test accurately detected those with amyloid plaques in their brains, showing equal accuracy in identifying amyloid presence in both symptomatic and asymptomatic individuals. Research has indicated that individuals without cognitive issues but positive for amyloid are at a high risk of developing cognitive impairments in the coming years.
“The accuracy of this blood test now enables us to diagnose the presence of Alzheimer’s disease pathology with a single blood sample,” said Randall J. Bateman, MD, the Charles F. and Joanne Knight Distinguished Professor of Neurology at Washington University. “This advance will increase accurate diagnoses for many patients.”
“In the near future, this type of blood test will replace the need for costly and less accessible cerebrospinal fluid and PET imaging tests in specialist memory clinics,” added Oskar Hansson, MD, PhD, a professor of neurology at Lund University. The researcher’s study was published in Nature Medicine on February 21, 2024.
12 LabMedica International April/2024
3-PART HEMATOLOGY ANALYZER GOLDSITE DIAGNOSTICS
MALARIA TEST HUMASIS
LMI-4-24 COM
LMI-4-24 LINKXPRESS COM
212
device WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile
Image: The blood test could make early Alzheimer’s diagnosis and treatment accessible to more people (Photo courtesy of 123RF)
State-of-the-Art Techniques to Investigate Immune Response in Deadly Strep A Infections
Annually, a staggering half a million people, including numerous children and young individuals, succumb to serious infections caused by the group A Streptococcus (Strep A) bacteria globally. Strep A is highly transmissible and spreads from person to person mostly via the respiratory route. Strep A is typically known for causing sore throats and skin infections in younger children. In rare cases, it can lead to more severe conditions like sepsis and toxic shock if the bacteria invade the bloodstream or tissue. While adults are often immune to Strep A sore throats and skin infections, both adults and children are very susceptible to the invasive form of the infection. A particularly alarming consequence of repeated Strep A infections is the autoimmune-induced damage to heart valves, termed rheumatic heart disease (RHD). RHD affects approximately 50 million people worldwide, predominantly in middle- and low-income countries. Currently, there is no vaccine available for Strep A. The development of immunity to Strep A over time, including the identification of specific bacterial antigens crucial for targeting by the immune system or future vaccines, remains poorly understood. Additionally, the distinction between detrimental immune overreactions to Strep A, leading to RHD,
ment of a vaccine that mimics and accelerates this immunity in children. Additionally, the iSpy-EXPLORE sub-network is set to explore the nature of protective immune responses in experimental models exposed to promising Strep A vaccine candidates. It will also assess human immune responses in healthy volunteers experimentally exposed to Strep A infections. Collectively, these efforts are poised to enhance understanding of both beneficial and detrimental immunity to Strep A, paving the way for a future vaccine that could protect against strep throat, invasive infections, and RHD.
“The RHD patients we see in LMICs generally present with advanced disease and complications such as heart failure,” said Professor Liesl Zuhlke, a pediatric cardiologist at The


USA; www.imperial.ac.uk), unites 28 researchers from 11 countries. This diverse group of experts in immunology, infectious disease, epidemiology, vaccinology, and experimental medicine will engage in a five-year project. Their goal is to utilize a broad spectrum of advanced techniques to delve into Strep A immunity with unprecedented detail, ultimately contributing significantly to reducing the global impact of Strep A.
The iSpy-LIFE sub-network aims to uncover how effective immunity to Strep A develops in children, following natural infection over time. This research, involving young children, school-age pupils, and adults, may provide insights into genuine immunity against Strep A and guide the develop-

University of Cape Town and iSpy team member. “Many require cardiac surgery or percutaneous intervention which are often not available, resulting in significant mortality and morbidity and incurring huge out-of-pocket costs to families and communities. We desperately need data on transitions between the various forms of Strep A diseases and how we can intervene to prevent these manifestations.”

For small and medium labs, @SINGUWAY is your answer.


≤30 minutes
Singu20 Nucleic Acid Extractor AccuRa-32 Real-Time PCR System (32 samples)




≤35 minutes
Singu20 Nucleic Acid Extractor AccuRa mini Real-Time PCR System (8-16 samples)
qPCR Detection Kits (PCR fluorescence method):
Respiratory Diseases: COVID-19, fluA, fluB, AdV, TB and multiplex test
Blood Diseases: HBV, HCV, HIV and multiplex test
Sexually Transmitted Diseases: HPV, CT, NG, UU and multiplex test
Viral Zoonotic Diseases: MPV
Vector-borne Diseases: PF, ZIKV
Genetic Diseases: MTHFR
Animal Diseases: Swine/Avian/Aquatic
Animal/Ruminant/Companion Animal Diseases





LabMedica International To view this issue in interactive digital magazine format visit www.LabMedica.com 13 LabMedica International April/2024 113 LMI-4-24 LINKXPRESS COM
5-Part Hematology Analyzer Hematology + CRP +SAA Joint Analyzer (Auto Sampling)


The ABSOL CARRY is a point-of-care Immunoassay System with microfluidics technology for providing accurate and stable diagnostics. Designed for POCT, the device provides quantitative results based on immunoassay technology. AUTOMATIC

Method Detects Pathogens in Blood Faster and More Accurately by Melting DNA
Cont’d from cover
sepsis cases is underscored by the fact that the mortality risk escalates by 4% every hour the infection is not properly identified or treated. Now, a new analysis technique offers quicker and more precise pathogen detection in blood samples compared to traditional blood cultures, which are the standard in infection diagnosis.
The new method, called digital DNA melting analysis, has been developed by researchers at UC San Diego (La Jolla, CA, USA; www. ucsd.edu) and is capable of delivering results in less than six hours. This marks a significant improvement over the typical 15 hours to several days required by culture methods, depending on the pathogen involved. The process utilizes universal digital high-resolution DNA melting, involving heating DNA until it separates. Each DNA sequence reveals a unique signature during the melting process. By imaging and analyzing this process, machine learning algorithms can discern the types of DNA in the samples and identify pathogens. This method not only outpaces blood cultures in terms of speed but also has a substantially lower risk of generating false positives compared to other emerging DNA detection technologies, such as Next Generation Sequencing.
The research began with one milliliter of blood from each of 17 patients in a preliminary clinical study. These samples were collected concurrently with those for blood cultures from infants and toddlers. The researchers honed the DNA isolation process and machine learning techniques to minimize or eliminate interference from human DNA in contrast to pathogen DNA in the samples. They refined a machine learning algorithm to accurately distinguish between the melting curves of pathogens and background noise. This algorithm correlates the observed curves with a database of known DNA melt curves. Moreover, it can identify curves produced by organisms not in this database, which is particularly useful in detecting rare or emerging pathogens in a sample.
The results from this method were not only consistent with those obtained from blood cultures of the same samples, but they also did not yield any false positives. This contrasts with other tests based on nucleic acid amplification and next-generation DNA sequencing databases, which tend to amplify all present DNA, leading to false positives. Contamination from various sources such as the environment, test tubes, reagents, and skin can often lead to challenges in interpreting test results. This new method detected pathogens 7.5 hours to approximately 3 days faster than conventional blood cultures. Additionally, it provides more than just a binary positive or negative outcome; it quantifies the extent of pathogen
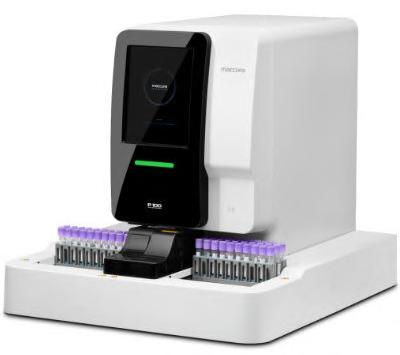
The P 100 automatic specific protein analyzer supports high-sensitivity C-reactive protein determination reagent. Adopting Immune Scatter Turbidimetry, it is suitable for a variety of application scenarios in medical laboratories.

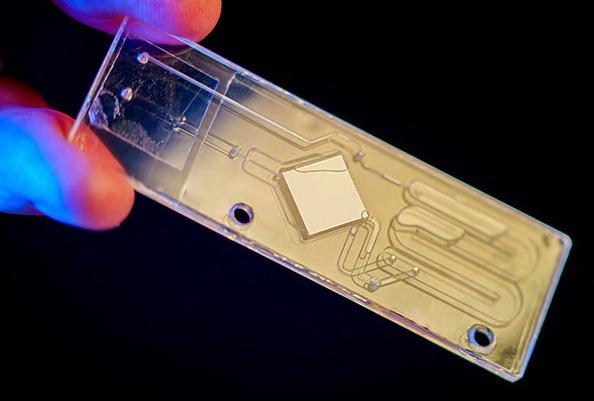
Image: Researchers used
presence in the samples. Future plans include conducting a more extensive clinical study and extending the methodology to adult patients.
“This is the first time this method has been tested on whole blood from patients suspected of having sepsis. So this study is a more realistic preview of how the technology could perform in real clinical scenarios,” said Stephanie Fraley, a professor at the UC San Diego. “We want to give doctors the ability to treat their patients based not on aggregate data, but with precise, accurate individual data, enabling truly personalized medicine.”
Sensitive Epigenetic-Based PCR Test Could Detect Difficult-to-Diagnose Breast Tumor
Phyllodes tumors, which make up less than 1% of breast tumors, present a diagnostic challenge due to their microscopic resemblance to other breast tumor types. While the majority of phyllodes tumors are benign, about 10% are malignant. Getting an accurate diagnosis is key to ensuring the right treatment is given, as a misdiagnosis can lead to inappropriate or delayed care. Typically, tumor diagnosis is based on the pathological examination of cellular patterns. Now, scientists have found the epigenetic ‘signature’ of phyllodes tumors, paving the way for the development of a sensitive epigenetic-based PCR test to detect this hard-to-diagnose breast tumor that could be routinely used in pathology laboratories.
Scientists at the Garvan Institute of Medical Research (NSW, Australia; www.garvan.org.au) have identified new DNA markers
Cont’d on page 15
14 LabMedica International April/2024
SYSTEM
POC IMMUNOASSAY
SPECIFIC PROTEIN ANALYZER MACCURA BIOTECHOLOGY
214 LMI-4-24 COM 215 LMI-4-24 LINKXPRESS COM
receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
To
this chip to analyze the microbes present in whole blood samples (Photo courtesy of UC San Diego)
Improved Microneedle Technology
Speeds Up Extraction of Sample Interstitial Fluid for Disease Diagnosis
Interstitial fluid has many similarities with blood, and its secrets are still being uncovered. A microneedle offers a minimally invasive method to sample this fluid directly under the skin. This tool allows for real-time and continuous monitoring of biomarkers circulating in the body. Despite their small size, just two to three times the width of a human hair and around a millimeter in length, microneedles can make a significant difference in early infection diagnosis and personal health monitoring. Now, researchers have developed improved microneedle technology that enhances the extraction of interstitial fluid by collecting more sample quantities in less time.
Sandia National Laboratories (Albuquerque, NM, USA; www.sandia. gov) is leading the way in microneedle research through collaboration with different partners to enhance this technology. Sandia has achieved a breakthrough in interstitial extraction, moving from using multiple needle arrays to a single microneedle technique that collects enough fluid for analysis in just about 10 minutes. This method is not only quicker but also gathers larger fluid volumes. The microneedles, designed to bypass nerve endings by not penetrating too deeply, are hollow and have been optimized by modifying the needle holders’ shape, which are 3D printed at Sandia’s Advanced Materials Laboratory.
This advancement could broaden microneedle applications significantly. For example, Sandia is exploring using microneedles to differentiate between bacterial and viral infections. This distinction could enable faster, more precise treatments. Additionally, Sandia is investigating the biomarkers present in interstitial fluid to see how they correlate with blood measurements. This research involves collecting interstitial fluid from volunteers using the new method, with the aim of developing devices for continuous health monitoring. Moreover, Sandia is also working on another project to develop microneedle sensors that detect electrolytes like sodium, potassium, and calcium. Continuous monitoring of these electrolytes could assist in managing cardiovascular functions, hydration levels, and electrolyte imbalances, offering benefits similar to a wearable glucose meter for various health conditions.
“When we started work in this field in 2011, our goal was to develop microneedles as a wearable sensor, as an alternate to blood samples,” said Ronen Polsky, who has led Sandia’s work in microneedles. “People wear continuous glucose monitors for blood sugar measurements. We want to expand this to a whole range of other conditions to take advantage of this minimally invasive sampling using microneedles.”
Sensitive Epigenetic-Based PCR Test Could Detect Difficult-to-Diagnose Breast Tumor
Cont’d from page 14
based on epigenetics that could provide additional diagnostic information for phyllodes tumors. Epigenetic alterations involve changes in gene activity levels without modifying the DNA sequence itself and can be influenced by environmental factors. One common epigenetic process is DNA methylation, where methyl groups attach to DNA segments, altering gene expression. In their study of samples from 33 patients, the research team observed a distinct DNA methylation pattern in phyllodes tumors, distinguishing them from other cancers. They also developed an algorithm that successfully reclassified samples that had initially been misdiagnosed. This advancement in understanding may lead to more accurate diagnoses and better patient outcomes.
“Disruption to epigenetic processes, such as DNA methylation patterns, is a recognized hallmark of cancer and can vary significantly between cancer types, allowing a unique cancer forensic signature,” said Professor Susan Clark, co-senior author and Head of the Cancer Epigenetics Lab at Garvan. “Harnessing the power of cutting-edge epigenetic technologies, like Digital Droplet PCR, our next step will be devising a sensitive epigenetic-based PCR test to detect phyllodes tumors that could be routinely used in pathology laboratories.”
















15 LabMedica International April/2024
LabMedica International To view this issue in interactive digital magazine format visit www.LabMedica.com
115 LMI-4-24 LINKXPRESS COM Size: 2400 x 1200 mm (3 mm thick) 100% Silicone YOUR GLOBAL SOURCE FOR STERILIZATION ACCESSORIES Thermo-Resistant (- 60 °C to 300 °C) Fully Washable & Flexible Suitable for central sterilization services Sterilizable STERILIZABLE INSTRUMENT & WORK-SURFACE MATS Front Back WASHING TRAYS MAT Heavy Silicone Cover & Transport Tablet TURBO WASHING MACHINES TRAYS SILICON INSTRUMENT MAT Front Back MICRO INSTRUMENT MAT Exchangable Net Exchangable Nets INVITEDTOAPPLY DISTRIBUTORS Up to 37 cm in length THERMO RESISTANT GLOVES Front Back WASHING TRAYS MAT NEW! VICOTEX Place de la Gare 1 • 1009 Pully • Switzerland Tel: (41) 21-728-4286 • Fax: (41) 21-729-6741 E-Mail: contact@vicotex.com www.vicolab.com S.A. SILICONE TABLET AND STEEL COVER NETS
Image: Microneedles measure about a millimeter long, as seen here on an extraction device (Photo courtesy of Craig Fritz)


Protein Biomarker to Help Develop Blood-Based Tests for Aggressive Neuroendocrine Carcinomas
Neuroendocrine carcinomas, such as neuroendocrine prostate cancer and small-cell lung cancer, originate in hormone-releasing cells and can develop in various organs, including the prostate and lungs. While they are not the most prevalent cancer type in these organs, they often have a poor prognosis and limited therapeutic options. Current treatments for these cancers include chemotherapy, radiation, and immunotherapy combinations. Neuroblastoma, predominantly found in young children, develops from immature nerve cells, often in the adrenal glands or nerve tissue along the spine, chest, abdomen, or pelvis. Despite treatment efforts, these therapies only extend survival by a few months, underscoring the need for better therapeutic targets and less invasive diagnostic approaches for these malignancies.
Investigators from the UCLA Health Jonsson Comprehensive Cancer Center (Los Angeles, CA, USA; www.uclahealth.org) have identified UCHL1, a protein found in aggressive neuroendocrine carcinomas and neuroblastoma, as a potential molecular biomarker for diagnosing these cancers and predicting and monitoring therapy responses. They also discovered that using a UCHL1 inhibitor, either alone or combined with chemotherapy,
Asignificantly delayed neuroendocrine carcinomas and neuroblastoma growth and spread in pre-clinical models. To find druggable targets for neuroendocrine carcinomas and neuroblastoma, the researchers first analyzed publicly available proteomics data and identified UCHL1 as one of the top druggable proteins.
UCHL1 levels in tissues from various neuroendocrine carcinoma patients revealed elevated levels in neuroendocrine prostate cancer, lung carcinoid, small-cell lung cancer, neuroblastoma, and other neuroendocrine neoplasms. This indicates that UCHL1 could be a common drug development target in neuroendocrine cancers due to its higher expression in these tumors compared to non-neuroendocrine tissues. The team then tested the therapeutic potential of blocking UCHL1 in pre-clinical models of neuroendocrine carcinomas and neuroblastoma. This research can help develop new minimally invasive bloodbased tests to detect and monitor therapy responses in patients with neuroendocrine carcinomas, such as highly aggressive neuroendocrine prostate cancer and small-cell lung cancer, and neuroblastoma. It also lays the foundation for new clinical trials to test UCHL1 inhibition as a new treatment approach that could help reduce deaths associ-
MAGNETIC BEAD SEPARATION MODULES
INTEGRA BIOSCIENCES

MAG and HEATMAG modules are designed for efficient, automated magnetic bead purification, and allow researchers to streamline several processing steps in molecular biology and proteomics workflows for maximum lab throughput.


Image: The protein UCHL1 could lead to a new therapeutic approach for managing aggressive neuroendocrine carcinomas (Photo courtesy of Stoyanova, et al., doi. org/10.1016/j.xcrm.2023.101381)
ated with aggressive diseases.
“Our study demonstrates the therapeutic potential of targeting UCHL1 and its utility as a detection tool in neuroendocrine carcinomas and neuroblastoma in pre-clinical models creating a critical translational link between the study and the diagnosis and treatment of patients with these malignancies,” said Dr. Tanya Stoyanova, associate professor of molecular and medical pharmacology and urology at the David Geffen School of Medicine at UCLA.
Novel Immunoassays Enable Early Diagnosis of Antiphospholipid Syndrome
ntiphospholipid syndrome (APS) is an autoimmune disorder that typically presents as venous or arterial thrombosis and/or pregnancy loss. Diagnosing APS can be difficult as its symptoms often resemble those of other diseases. Prompt diagnosis is essential to avoid complications, unnecessary medical procedures, and escalating healthcare
costs. Now, a new pair of reagents can enable early diagnosis of APS, one of the hard-to-diagnose autoimmune diseases.
Werfen’s (Barcelona, Spain; www.werfen. com) Aptiva APS Immunoglobulin G (IgG) and Immunoglobulin M (IgM) reagents are immunoassays that utilize Aptiva particle-based multi-analyte technology (PMAT) for the
semi-quantitative determination of anti-cardiolipin (aCL) and anti-beta 2 glycoprotein 1 (aβ2GP1) IgG and IgM autoantibodies in human serum and citrated plasma. They serve as a diagnostic aid for both primary and secondary APS, in conjunction with other laboratory findings.
Cont’d on page 20
16 LabMedica International April/2024
LUNG CANCER TEST AGENA BIOSCIENCE
The iPLEX HS Lung Panel enables tumor profiling studies of NSCLC specimens and can detect 70 clinically relevant variants. It can identify variants at as low as 1% variant allele frequency using as little as 10
217 LMI-4-24 COM 218 LMI-4-24 LINKXPRESS COM To receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
Edited by Katherina Psarra, MSc, PhD
MESSAGE FROM THE PRESIDENT
By Tomris Ozben • President, IFCC
Dear Colleagues and Friends,
I am delighted to present an overview of the recent accomplishments and significant developments of our Federation, starting with the IFCC Executive Board meetings: these meetings are instrumental in shaping our strategic direction and ensuring the continued growth and success of our federation.
Our Executive Board convened in a hybrid format on February 14th, bringing together the Chairs of the four Divisions, the Congresses and Conferences Committee, and nine Task Forces. The agenda encompassed comprehensive discussions and reception of activity reports until December 2023. Noteworthy was a separate deliberation with the Task Force on Global Lab Quality to delineate future plans, reflecting our commitment to continuous improvement. Continuing our momentum, a pivotal meeting took place in Milan on February 15th. Deliberations centered on IFCC Strategic Action Plans for the term (2024-2026), with a focus on areas crucial for our collective advancement. This encompassed supporting membership, broadening horizons, enhancing the quality of laboratory medicine, and improving the effectiveness of IFCC operations. The IFCC Strategic Action Plans will be jointly developed by the IFCC Executive Board, IFCC Functional Units, IFCC Member Societies, Regional Federations and Corporate Members. The outcomes of these discussions will be finalized for presentation at the upcoming Council meeting, scheduled as a hybrid event on Sunday, May 26th, coinciding with the WorldLab Congress in Dubai.
A significant achievement during this period was the meticulous selection of scholarship recipients for the WorldLab Congress. With a remarkable number of applications received, our selection process adhered to stringent criteria to ensure fairness and meritocracy. The benefits provided to the recipients will empower them in their academic pursuits and professional endeavors, contributing to the advancement of clinical chemistry and laboratory medicine globally.
Preparations for the YS (Young Scientists) Forum and IFCC Global MedLab Week 2024 are well underway. These initiatives play a vital role in nurturing talent and fostering collaboration among young professionals in our field. From program definition to logistical arrangements to the launch of the official logo,
these events will ensure a memorable impact that reflects the innovation of our organization. Additionally, discussions on the implementation of IFCC Professional Exchange Programmes (PEP) and budget considerations underscore our dedication to operational excellence and sustainability.


Similar to other important activities that already show our commitment towards our strategic objectives, we signed a Memorandum of Understanding (MoU) with the Fédération Internationale Francophone de Biologie Clinique et de Médecine de Laboratoire (FIFBCML),



www.dubai2024.org



The Congress is accredited!
IFCC is an approved EFLM CPECS® Provider of Continuing Education Events in Laboratory Medicine. The IFCC Worldlab 2024 has been accredited by the EFLM-CPECS® credit system for a maximum number of 22,5 CPECS® credits.
NEWS
IFCC members may send news to
17 LabMedica International April/2024
Email: enews@ifcc.org
26th International Congress of Clinical Chemistry and Laboratory Medicine
Dubai World Trade Centre (DWTC)
In cooperation with Cont’d on page 18

Global Recognition Awaits: Get Started Today!
Enhanced patient safety. Improved wellness. Enhanced equity. Mitigated costs. Enhanced clinical confidence, and more. These key performance indicators (KPIs) are representative outcomes that laboratory medicine and associated insights can and do impact every day. If you are a passionate healthcare professional who seeks to collaborate, innovate and measure improvements through strategic initiatives, then now is the time to start preparing your application to the UNIVANTS of Healthcare Excellence award program.

The UNIVANTS of Healthcare Excellence award program is global, prestigious award program that, through 8 prestigious program partners, including Abbott, International Federation of Clinical Chemistry and Laboratory Medicine (IFCC), Association for Diagnostics and Laboratory Medicine (ADLM, formerly branded as AACC), Modern Healthcare, National Association for Healthcare Quality (NAHQ), European Health Management Association (EHMA), Institute of Health Economics (IHE), Healthcare Information and Management Systems Society (HIMSS), seeks to recognize, amplify, and celebrate best practices in healthcare that are facilitated by laboratory medicine.
To be eligible for potential recognition, submitted application must meet the below eligibility criteria:
Cont’d from page 17
1) The clinical care initiative must be implemented into clinical practice.
2) The clinical care initiative must include at least three disciplines (including Laboratory Medicine/Pathology).
3) There must be at least one measurable impact or Key Performance Indicator (KPI) associated with each of the four stakeholders: patients, clinicians, health systems/ administration, and payors.
4) Impact can be assessed quantitatively (preferred) or qualitatively, but there must be at least two quantitative metrics and no more than four qualitative metrics.
Applications for the 2025 UNIVANTS of Healthcare Excellence awards open Aug 1, 2024.
What can you do right now?:
.Start thinking about your application.
.Visit www.UnivantsHCE.com for details on how to apply, as well as tips and tricks for maximizing your application.
.Begin collating metrics.
.Reach out to UnivantsOfHealthcareExcellence@abbott. com with any questions.
For more information on past winners, best practice examples and to tune in for the Global Announcement of the 2024 UNIVANTS of Healthcare Excellence award program winners (in June 2024), please visit www.UnivantsHCE.com.
Message from the President
underscoring our commitment for global collaboration.
Looking ahead, we are poised to strengthen our partnerships with the BIPM across various domains, including traceability in laboratory medicine and metrology standards. Furthermore, discussions on collaboration with the IUPAC and SNOMED for the development of healthcare terminology highlight our proactive attitude in enhancing industry standards and interoperability.
On a more personal note, I extend my heartfelt appreciation to the EMD-VLP Committee, its Chair Prof. Sedef Yenice, and Abbott for enabling my participation as a visiting lecturer at the 3rd Annual International Laboratory Quality and Accreditation FORUM. Hosted in collaboration with AFCC and the Egyptian Association of Healthcare Quality and Patient Safety, the Egylabs FORUM was held in Cairo on February 28-29, 2024. I delivered the Opening Lecture on “Green laboratories: Implementing sustainable practices in medical laboratories,” emphasizing the vital role of laboratory medicine in public health, environment and sustainability. At the event, I chaired sessions and spoke on the importance of accreditation and quality assessments, fostering collaboration between IFCC and AFCC societies to elevate laboratory standards across Africa and to achieve minimum quality requirements in medical laboratories.
Before closing this message, once again I extend my invitation to you to join us at the 26th International Congress of Clinical Chemistry and Laboratory Medicine (ICCCLM) - IFCC WorldLab Congress. This prestigious event will be held jointly with the 17th Congress of the Arab Federation of Clinical Biology (AFCB), the 10th Annual Meeting of the Saudi Society for Clinical Chemistry (SSCC), and the 8th International and UAE Genetic Disorders Conference in partnership with MZ Events, taking place at the Dubai World Trade Centre (WTC) from May
26-30, 2024. Additionally, the 3rd IFCC FORUM for Young Scientists is planned to precede the congress.
The IFCC WorldLab Congress serves as a global platform for the exchange of knowledge and expertise in clinical chemistry and laboratory medicine across academic, clinical, and industrial domains. It fosters interaction among clinical laboratory scientists and physicians worldwide, promoting advancements in human health. The Scientific Program Committee is dedicated to curating a multidisciplinary program covering fundamental concepts, advanced diagnostics, and emerging techniques in laboratory medicine. This program will feature plenary lectures, symposia on cutting-edge topics, oral presentations, posters, and educational workshops, providing ample opportunity for learning, discussion, and collaboration.
The In Vitro Diagnostic (IVD) Sector will host an extensive exhibition showcasing the latest technological innovations and practical solutions tailored to the needs of clinical laboratories. Additionally, high-level educational workshops led by esteemed speakers will cover various disciplines pertinent to laboratory medicine, further enriching the congress experience.
I eagerly anticipate the participation of IFCC members from across the globe, representing six IFCC Regional Federations, in this exciting WorldLab Congress hosted by the Arab Federation of Clinical Biology. I am confident that your attendance will be both rewarding and enriching, offering inspiring scientific sessions, engaging discussions, networking opportunities, and memorable social activities.
In closing, I extend my gratitude to the dedicated members of IFCC for their unwavering commitment and enthusiasm. Together, we continue to chart a course towards excellence in clinical chemistry and laboratory medicine, fostering innovation, collaboration, and impactful change on a global scale.
News from the World of the International Federation of Clinical Chemistry and Laboratory Medicine Visit www.ifcc.org for more information NEWS 18 LabMedica International April/2024

Join Us in Celebrating Success of the IFCC GMLW 2024!
For the third consecutive year, the IFCC invited clinical laboratory professionals worldwide to participate in the GLOBAL MEDLAB WEEK 2024 festivities. This year’s theme, “Laboratories Save Lives,” resonates with our shared experiences and highlights the critical role laboratories play in healthcare. The GMLW 2024 took place from April 22 to 28.
We accepted submissions of audio recordings and videos, ranging from 1 to 4 minutes in length. IFCC provided comprehensive guidelines for submission.

The IFCC Committee on Public Relations (C-PR) spearheaded this event in collaboration with the IFCC Task Force for Young Scientists (TF-YS), receiving invaluable support from National Representatives and Champions of IFCC
member countries, as well as Regional Representatives of the 6 IFCC Regional Federations: African Federation of Clin ical Chemistry (AFCC), Arab Federation of Clinical Biology (AFCB), Asia-Pacific Federation for Clini cal Biochemistry and Laboratory Medicine (APFCB), European Federation of Clinical Chemistry and Labora tory Medicine (EFLM), and Latin-American Confederation of Clinical Biochemistry (COLABIOCLI).
Prizes were awarded to the first, second, and third place winners for the best videos. Moreover, all professionals who contributed to the event, whether through audio or video submissions, received an IFCC participation certificate as a token of appreciation for their collaboration.
We extend our gratitude to the coun-
tries that submitted materials for podcasts and videos. Throughout GMLW, scientific societies organized various academic and recreational activities, newsletters, and interviews on radio and television. Let’s continue to raise awareness about the indispensable role of clinical laboratory professionals in patient healthcare because “Laboratories Save Lives.”
Spread the word about IFCC GMLW 2024 on your social networks and visit all IFCC social media platforms to be part of this remarkable week, promoting the visibility of laboratory medicine.
Visit us at: https://globalmedlabweek.org
The New IFCC eAcademy Website
We are pleased to announce the launch of the IFCC eAcademy website, offering a diverse array of educational resources for professionals in the field. With a wealth of over 70 webinars and 200 presentations curated by outstanding speakers across a wide range of scientific topics of scientific topics, the IFCC eAcademy provides an enriching learning experience for all. Participants have the opportunity to deepen their knowledge through engaging webinars and gain exclusive certificates of participation upon completion. Additionally, the IFCC eAcademy features IFCC VLP lectures from the IFCC-Abbott Visiting Lecturer Programme (VLP), as well as Pearls, dedicated to the Spanish-speaking audience, and Podcasts filled with valuable insights from renowned experts in various fields
of the Laboratory.
Furthermore, visitors can explore our dedicated author section to connect with the experts behind the content and learn from their vast expertise and accomplishments.
We invite you to join us on the IFCC eAcademy platform and embark on a journey of continuous learning and professional development.
Visit the eAcademy: eacademy.ifcc.org Register to the eAcademy (eacademy. ifcc.org/registration) and benefit from all its educational opportunities.


19 LabMedica International April/2024 News from the World of the International Federation of Clinical Chemistry and Laboratory Medicine
www.ifcc.org for more information NEWS The views and positions expressed in the IFCC News section are those of the IFCC or the individual authors, and do not necessarily represent the views or positions of LabMedica magazine or its publishers.
OFFICE
Visit
IFCC
Via Carlo Farini 81, 20159 Milan, ITALY Tel: (39) 02-6680-9912
E-mail: ifcc@ifcc.org • Web: www.ifcc.org
Staff Members: Paola Bramati, Silvia Cardinale, Silvia Colli-Lanzi, Elisa Fossati, Smeralda Skenderaj

The Quick Profile Vitamin D is designed for the quantitative or semi-quantitative determination of total 25-hydroxy Vitamin D (25-OH Vitamin D) in human blood. It is available as one step rapid test and EIA.

Portable Rapid PCR Diagnostic to Detect Gonorrhea and Antibiotic Susceptibility
Gonorrhea, ranked as the second most reported bacterial sexually transmitted infection (STI), affected approximately 82 million people globally in 2020. The infection can lead to severe health complications, including pelvic inflammatory disease, chronic pelvic pain, and infertility. Untreated gonorrhea may progress to the bloodstream, posing a life-threatening risk and increasing the likelihood of HIV infection. Many cases go unreported due to asymptomatic patients, implying that the actual burden of the disease may be significantly higher. Now, a rapid test aims to identify gonorrhea and also determine its antibiotic susceptibility.
Visby Medical (San Jose, CA, USA; www. visbymedical.com) has been awarded up to USD 1.8 million by CARB-X (Boston, MA, USA; carb-x.org) for the development of a portable rapid polymerase chain reaction (PCR) diagnostic to identify the presence of Neisseria gonorrhoeae (NG), the pathogen responsible for gonorrhea, and ascertain its susceptibility to ciprofloxacin. Ciprofloxacin was once a primary oral antibiotic for treating NG but has become less effective due to resistance. A rapid result indicating when ciprofloxacin may be effective would allow physicians to treat gonorrhea with greater confidence, reserving ceftriaxone for
cases resistant to NG. Visby is renowned for its rapid and precise PCR testing capabilities for STIs, as well as COVID-19 and the flu. The funding from CARB-X will assist Visby in advancing to the next phase of development to address the pressing challenges in today’s healthcare system.
Besides the development of a rapid test for NG and its susceptibility to ciprofloxacin, the funding will help Visby develop a test for NG as well as Chlamydia trachomatis (CT) and Trichomonas vaginalis (TV) in men based on urine samples. Visby currently offers a second-generation Sexual Health Test for the three most prevalent STIs in women, which is 510(k) cleared and has received a CLIA waiver from the U.S. Food and Drug Administration. Further CARB-X funding will be allocated in separate phases, contingent upon achieving specific project milestones, covering feasibility testing and development for antimicrobial resistance and male STI testing applications.
“The sexually transmitted infections epidemic continues to increase. That is why healthcare providers in ERs, urgent care clinics, community health centers and physicians’ offices need accurate and rapid diagnostic tests to enable same-visit, data-driven treatment based on a test result that identifies the pathogen and its antibiotic susceptibility,”
The Magicplex Sepsis Real-time Test is a comprehensive assay for detection and identification of sepsis-causing pathogens. It can screen for more than 90 pathogens (over 90% of sepsis-causing pathogens) from whole blood samples.


Image: Visby Medical currently offers a second generation Sexual Health Test for the three most common STIs in women (Photo courtesy of Visby)
said Gary Schoolnik, MD, Chief Medical Officer of Visby Medical. “The CARB-X award will allow Visby Medical to enhance the only instrument-free PCR platform by adding the capacity to detect ciprofloxacin-resistant gonorrhea immediately and at the point-ofcare. This has the potential to redefine best practice for patient care, infection control, and antibiotic stewardship.”“Our goal is to deliver diagnostics that are effective at all levels of the healthcare system,” added Erin Duffy, PhD, R&D Chief of CARB-X. “Given the portability of the envisioned Visby Medical PCR platform, which fits in the palm of your hand, we see this as rapidly and highly deployable in low-resourced and hard-to-reach settings. Additionally, for regions where ciprofloxacin remains a viable treatment, the Visby Medical diagnostic gives confidence that the physician is making the correct treatment decision.”
Novel Immunoassays Enable Early Diagnosis of Antiphospholipid Syndrome
Cont’d from page 16
The Aptiva system is a fully automated, multi-analyte system, representing the latest advancement in high throughput analyzers for autoimmunity and immunology laboratories. Utilizing PMAT, Aptiva can process up to 120 APS tests per hour, allowing laboratories to handle their workload more efficiently and with reduced manual inter-
vention. Werfen has recently announced that it has received the CE (Conformité Européenne) mark for its Aptiva APS IgG and IgM reagents. These new reagents not only complement Werfen's existing Aptiva Celiac Disease and Connective Tissue Diseases (CTD) Essential reagents but also extend the range of CE Marked analytes detectable by Aptiva to a total of 19.
"Early diagnosis is crucial in preventing complications as well as unnecessary procedures and increased healthcare costs,” said Michael Mahler, PhD, Vice President of Research and Development at Werfen. “Aptiva APS IgG and APS IgM deliver expanded information to clinicians to help with the diagnosis and management of patients with autoimmune diseases."
20 LabMedica International April/2024
VITAMIN D TEST LUMIQUICK DIAGNOSTICS
SEEGENE
SEPSIS TEST
220 LMI-4-24 COM 221 LMI-4-24 LINKXPRESS COM To receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
Beckman Coulter and Fujirebio Expand Partnership on Neurodegenerative Disease Diagnostics
Beckman Coulter Diagnostics (Brea, CA, USA; www.beckman coulter.com) and Fujirebio Diagnostics (Tokyo, Japan; www. fujirebio.com) have expanded their partnership focused on the development, manufacturing and clinical adoption of neurodegenerative disease assays. Both companies entered into a collaboration agreement in 2023 to target new biomarkers in line with the recently approved monoclonal antibody-based Alzheimer’s disease therapeutics. These new drugs have demonstrated a major impact on slowing disease progression and improving patients’ health. The companies have now extended this research program and further refined the target analytes, including a pTau217 plasma assay being developed by Beckman Coulter and a Beta Amyloid 1-42 plasma assay under development by Fujirebio.
These new assays are being developed for use on the recently launched DxI 9000 Immunoassay Analyzer from Beckman Coulter. The DxI 9000 Analyzer’s novel Lumi-Phos PRO Substrate has demonstrated the ability to develop highly sensitive and clinically relevant assays. This agreement leverages Fujirebio’s expertise in the development, manufacturing and regulatory registration of its Neurological Reagent Kits and its wide Neurological Biomarker menu. Fujirebio currently offers a full range of research use only (RUO) bloodbased neurodegenerative biomarkers in-
ECCMID Congress to Rebrand as ESCMID Global
Over the last few years, the European Society of Clinical Microbiology and Infectious Diseases (ESCMID, Basel, Switzerland; escmid.org) has evolved remarkably. The society is now stronger and broader than ever before in the four decades since it was founded. Currently, ESCMID is focusing on stepping up the fight against antimicrobial resistance, advancing preparedness for emerging infections, and leading in the practice, education, and training of specialists. In recent years, ESCMID has welcomed over 4000 new members and expects to see a record-breaking 20,000 colleagues attend its congress in Barcelona. Hence, the organization feels the need to adapt to this new reality.
In 2023, ESCMID’s in-depth analysis of its brand and communication efforts revealed that the brand failed to adequately reflect ESCMID’s expanding global role. The analysis also revealed that most community members were unaware of various activities undertaken by ESCMID related to research, education, and guidelines. Additionally, most of its audience was unaware of the connection between the European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) and ESCMID. As a result, the society has now decided to strengthen its main brand, ESCMID, and bring all of its various sub-brands more closely together under one large ESCMID umbrella. This includes changing the name of its congress to “ESCMID Global”, creating a clear link to ESCMID, and conveying its outreach and perspective beyond Europe.

cluding β-amyloid1-42, β-amyloid1-40, phospho-Tau181, neurofilament light (NfL), ApoE4, Pan-ApoE and phospho-Tau217.
SMART MOVE.
REGISTER EARLY + SAVE
Time to put those keen powers of observation and evaluation into action and make your move. The earlier you register, the more you’ll save!
Join us in Chicago for a once in a lifetime meeting of the minds that will unite global laboratory medicine leaders for:
• Five plenaries, 65+ scientific sessions, and 170 roundtables
• 12 ADLM University courses offering practical skills for laboratorians
• 900+ exhibitors, 200+ product categories and live demos
• Networking opportunities to connect with industry peers and leaders
• And much more!
Register early before rates go up! meeting.myadlm.org


21 LabMedica International April/2024 Industry News To view this issue in interactive digital magazine format visit www.LabMedica.com
Image: These new assays are being developed for use on the recently introduced DxI 9000 Immunoassay Analyzer (Photo courtesy of Beckman Coulter)
JU LY 28–AUGUST 1 • CHICAG O, IL, US A JU LY 28–AUGUST 1 • CHICAG O, IL, US A
Jingcai Wang MD, PhD, NRCC, SH(ASCP),
MLS
For a free listing of your event or a paid advertisement contact:
LabMedica International Calendar
E-mail: info@globetech.net
2024
APRIL
Analytica 2024. Apr 9-12; Munich, Germany; analytica.de
India Lab Expo & Analytica Anacon India. Apr 15-17; Mumbai, India; analyticaindia.com
Korea Lab 2024. Apr 23-26; Seoul, Korea; korealab.org
ExpoMED Eurasia 2024. Apr 25-27; Istanbul, Turkey; expomedistanbul.com
ESCMID Global 2024 – 34th European Congress of Clinical Microbiology and Infectious Diseases. Apr 27-30; Barcelona, Spain; eccmid.org
The 15th International & 21st National Congress on Quality Improvement in Clinical Laboratories. Apr 30 - May 3; Tehran, Iran; iqctehran.ir
MAY
24th National Congress of Clinical Chemistry and Laboratory Medicine. May 2-5; Playa del Carmen, Mexico; fenacqc.org.mx
Immunology 2024 – Annual Meeting of the American Association of Immunologists (AAI). May 3-7; Chicago, IL, USA; immunology2024.aai.org
AACE Annual Meeting 2024 – American Association of Clinical Endocrinology. May 9-11; New Orleans, LA USA; pro.aace.com
ECE 2024 – 26th Annual Congress of the European Society of Endocrinology. May 11-14; Stockholm, Sweden; ese-hormones.org
ASRI 2024 – 43rd Metting of the American Society for Reproductive Immunology. May 18-22; Houston, TX, USA; theasri.org
Hospitalar 2024. May 21-24; Sao Paulo, Brazil;
hospitalar.com
107th Annual Meeting of the German Society for Pathology. May 23-25; Munich, Germany; pathologie-dgp.de
IFCC WorldLab 2024 – 26th International Congress of Clinical Chemistry and Laboratory Medicine. May 2630; Dubai, UAE; dubai2024.org
SLAS Europe 2024 Conference and Exhibition - Society of Laboratory Automation and Screening. May 27-29; Barcelona, Spain; slas.org
ISLH 2024 – International Society for Laboratory Hematology. May 30 - Jun 1; Nantes, France; islh.org
EAACI 2024 – Annual Congress of the European Academy of Allergy & Clinical Immunology. May 31Jun 3; Valencia, Spain; eaaci.org
JUNE
ESHG 2024 – European Human Genetics Conference. Jun 1-4; Berlin, Germany; 2024.eshg.org
UKMedLab24 – National Meeting of the Association for Clinical Biochemistry and Laboratory Medicine. Jun 10-12; Brighton, UK; acb.org.uk
9th International Symposium on Critical Care Testing and Blood Gases. Jun 13-14; Saint-Malo, France; criticalcaretesting-saintmalo2024.eu
ASM Microbe 2024 – American Society for Microbiology. Jun 13-17; Atlanta, GA, USA; asm.org
49th CBAC – Congress of the Brazilian Society of Clinical Analysis. Jun 16-19; Natal, Brazil; sbac.org.br
2024 CSCC Annual Conference – Canadian Society of Clinical Chemists. Jun 17-20; Mont Sainte-Anne, QC, Canada; cscc-sccc.ca
FOCIS 2024 – Annual Meeting of the Federation of Clinical Immunology Societies. Jun 18-21; San Francisco, CA, USA; focisnet.org
FIME 2024 – Florida International Medical Expo. Jun 19-21; Miami, FL, USA; fimeshow.com
ISTH 2024 Congress – International Society on Thrombosis and Haemostasis. Jun 22-26, Bangkok,
Thailand; isth2024.org
ECC 2024 – 45th European Congress of Cytology. Jun 23-26; Leipzig, Germany; cytology2024.eu
38th International Congress of the International Society of Blood Transfusion (ISBT). Jun 23-27; Barcelona, Spain; isbtweb.org
AMP Europe 2024. Jun 24-26; Madrid, Spain; amp.org ASV 2024 – 43rd Annual Meeting of the American Society of Virology. Jun 24-28; Columbus, OH, USA; asv.org
FEBS 2024 – 48th Congress of the Federation of European Biochemical Societies. Jun 29 - Jul 3; Milan, Italy; febs.org
ECB 2024 – European Congress on Biotechnology. Jun 30 - Jul 3; Maastricht, The Netherlands; ecb2024.com
JULY
ESHRE 2024 – 40th Annual Meeting of the European Society of Human Reproduction and Embryology. Jul 7-10; Amsterdam, Netherlands; eshre.eu
MedLab Asia 2024. Jul 10-12; Bangkok, Thailand; medlabasia.com
2024 ADLM Annual Scientific Meeting & Clinical Lab Expo. Jul 28 - Aug 1; Chicago, IL, USA; meeting. myadlm.org
AUGUST
64th Annual Academic Assembly of the Japan Society of Clinical Chemistry (JSCC). Aug 30 - Sep 1; Utsunomiya, Japan; jscc-jp.gr.jp
SEPTEMBER
ASCP 2024 – Annual Meeting of the American Society for Clinical Pathology. Sep 4-6; Chicago, IL, USA; ascp.org
17th Baltic Congress of Laboratory Medicine. Sep 57; Vilnius, Lithuania; balm2024.lt
ECP 2024 – 35th Congress of the European Society of Pathology. Sep 7-11; Florence, Italy; esp-congress.org
Events Calendar
Provided as a service to advertisers. Publisher cannot accept responsibility for any errors or omissions. Advertising Index Inq.No. Advertiser Page Inq.No. Advertiser Page Vol. 41 No. 2 4/2024 LabMedica International ATTENTION: IF YOUR APPLICATION IS NOT RECEIVED AT LEAST ONCE EVERY 12 MONTHS YOUR FREE SUBSCRIPTION MAY BE AUTOMATICALLY DISCONTINUED READER SERVICE PORTAL LINKXPRESS COM ® VISIT Every advertisement or product item contains a LinkXpress number as below: 999 LMI-04-24 LINKXPRESS COM Identify LinkXpress codes of interest as you read magazine Click on LinkXpress.com to reach reader service portal Mark code(s) of interest on LinkXpress inquiry matrix 1 2 3 Renew/ S tart Your Free Subscription Access Instant Online Product Information – ADLM 2024 21 – APFCB 23 – BCLF 2024 19 105 DiaSys Diagnostic Systems 5 – EuroMedLab 2025 23 – IFCC WorldLab 2024 17 109 IncreCare 9 111 InTec Products 11 – LabMedica EXPO 2 103 Randox 3 113 Singuway 13 107 Snibe 7 115 Vicotex 15 124 Werfen 24
EUROTOX 2024 – 58th Congress of the European Societies of Toxicology. Sep 8-11; Copenhagen, Denmark; eurotox2024.com
Thailand LAB International 2024. Sep 11-13; Bangkok, Thailand; thailandlab.com
NFKK 2024 – 39th Nordic Congress of Clinical Chemistry. Sep 17-20; Stockholm, Sweden; nfkk2024.se
ESVC 2024 – Annual Meeting for the European Society for Clinical Virology. Sep 17-21; Frankfurt, Germany; escv.eu
47TH ISOBM CONGRESS – International Society of Oncology and Biomarkers. Sep 19-21; Pilsen, Czech Republic; isobm.org
DKLM 2024 – Annual Congress of the German Society for Clinical Medicine and Laboratory Medicine (DGKL). Sep 25-27; Bremen, Germany; dgkl.de India Lab Expo & Analytica Anacon India. Sep 26-28; Hyderabad, India; analyticaindia.com
OCTOBER
COLABIOCLI 2024 - 26th Latin American Congress of Clinical Biochemistry. Oct 3-6; Cartagena, Colombia; colabiocli.com
56th National Congress of the Italian Society of Clinical Biochemistry and Clinical Molecular Biology (SIBioC). Oct 8-10; Bologna, Italy; sibioc.it
JFBM 2024 – Journées Francophones de Biologie Médicale. Oct 9-11; Troyes, France; jfbm.fr
33rd WASPaLM World Congress – World Association of Societies of Pathology and Laboratory Medicine. Oct 16-20; Antalya, Turkey; waspalm-association.org
CAP24 – Annual Meeting of the College of American
Pathologists. Oct 19-22; Las Vegas, NV, USA; cap.org
ASHI 2024 – 50th Annual Meeting of the American Society for Histocompatibility and Immunogenetics. Oct 21-24; Anaheim, CA, USA; ashi-hla.org
MedLab Africa 2024. Oct 22-24; Johannesburg, South Africa; africahealthexhibition.com
BCLF 2024 – 31st Meeting of the Balkan Clinical Laboratory Federation & 35th National Congress of the Turkish Biochemical Society. Oct 28 - Nov 1; Antalya, Turkey; turkbiyokimyadernegi.org.tr
APFCB Congress 2024 – Asia Pacific Federation for Clinical Biochemistry and Laboratory Medicine. Oct 31 - Nov 3; Sydney, Australia; apfcbcongress2024.org NOVEMBER
ALACI 2024 – 14th Latin American and Caribbean Immunology Congress. Nov 4-8; Buenos Aires, Argentina; alaci.org
ASHG 2024 – Annual Meeting of the American Society of Human Genetics. Nov 5-9; Denver, CO, USA; ashg.org
JIB 2024 – Journées de l’innovation en biologie. Nov 7-8; Paris, France; jib-innovation.com
72nd Annual Scientific Meeting of the American Society of Cytopathology (ASC). Nov 7-10; Orlando, FL, USA; cytopathology.org
46 Annual ACBI Conference 2024 – Association of Clinical Biochemists in Ireland. Nov 8-9; Dublin, Ireland; acbi.ie
MEDICA 2024. Nov 11-14; Dusseldorf, Germany; medica-tradefair.com
45th Annual Meeting of the American College of Tox-

icology (ACT). Nov 17-20; Austin, TX, USA; actox.org
Analytica China 2024. Nov 18-20; Shanghai, China; analyticachina.com.cn
AMP 2024 – Annual Meeting & Expo of the Association for Molecular Pathology. Nov 21-23; Vancouver, BC, Canada; amp.org
ADLM Middle East 2024. Nov 23-24; Dubai, UAE; adlmme.org
DECEMBER
ICID 2024 – 20th International Congress on Infectious Diseases. Dec 3-6; Cape Town, South Africa; isidcongress.org
ACBICON 2024 – 50th Annual Conference of the Association of Clinical Biochemists of India. Dec4-7; Chandigarh, India; acbicon2024.com
66th ASH Annual Meeting and Exposition – American Society of Hematology. Dec 7-10; San Diego, CA, USA; hematology.org
2025
JANUARY
SLAS 2025 – International Conference & Exhibition of the Society of Laboratory Automation and Screening. Jan 25-29; San Diego, CA, USA; slas.org
FEBRUARY
Medlab Middle East 2025. Feb 3-6; Dubai, UAE; medlabme.com
Labquality Days 2025 – International Congress on Quality in Laboratory Medicine. Feb 6-7; Helsinki, Finland; labqualitydays.fi

Events Calendar
23 LabMedica International April/2024

HIT Testing in Minutes.
The on-demand solution that saves more than time.
Fast, accurate HIT antibody detection. Prompt detection of HIT antibodies is critical to selection of the most appropriate therapy. Only Werfen provides a fully automated HIT assay on Hemostasis testing systems, ready-to-use, 24 hours/day, 7 days/week. Complete HIT testing solutions—now on-demand for ACL TOP® testing systems.
For more information, contact your local Werfen representative. werfen.com
132 LMI-4-23 LINKXPRESS COM Heparin-Induced Thrombocytopenia
ACL, ACL AcuStar, ACL Elite, ACL TOP, HemosIL, ReadiPlasTin, RecombiPlasTin, SynthAFax and SynthASil are trademarks of Instrumentation Laboratory Company and/or one of its subsidiaries or parent companies and may be registered in the United States Patent and Trademark Office and in other jurisdictions. The Werfen logo is a trademark of Werfen and may be registered in the Patent and Trademark Offices of jurisdictions throughout the world. ©2021 Instrumentation Laboratory. All rights reserved. 124 LMI-4-24 LINKXPRESS COM
























































































































